Diversity of arbuscular mycorrhizal fungi and Soil Physicochemical Parameters in Forest Species of the Abras de Mantequilla Wetland, Ecuador
DOI:
https://doi.org/10.17268/sci.agropecu.2025.001Palabras clave:
Sustainable, mycorrhizae, fertility, diversity, species, abundanceResumen
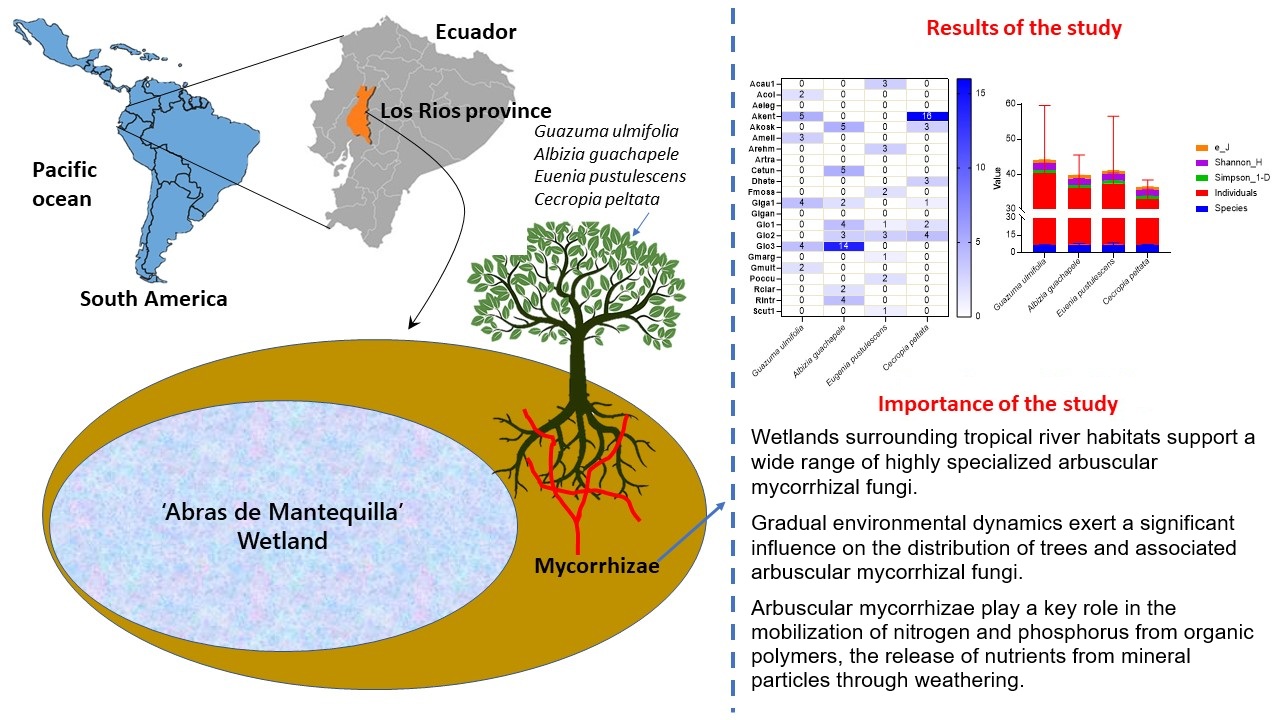
Wetlands worldwide face increasing challenges due to human activities such as water extraction, deforestation, and overfishing. These ecosystems are crucial for biodiversity, water retention, and purification. In Ecuador, the “Abras de Mantequilla” wetland exemplifies these pressures. It serves as a vital site for studying the interactions between soil fertility and arbuscular mycorrhizal fungi (AMF) in forest ecosystems. The study focused on the soil fertility, particularly in relation to AMF in "Noah Morán" secondary forest within the Abras de Mantequilla wetland. The key role of AMF in improving soil fertility was highlighted. Soil samples were collected from the root zone of four forest tree species i.e., Guazuma ulmifolia, Albizia guachapele, Eugenia pustulescens, Cecropia peltate. The four investigated soil samples had marginal differences in the soil physicochemical properties. Number of AMF species were found in the four soils in the range of 16 to 39; Number of por Several AMF lowest por the lowest (16) and highest (39) AMF species were found in E. pustulescens and A. guachapele soils, respectively. AMF species ‘Akent’ was the most dominant (abundance #16) AMF species found in the soils of C. peltate. The ranges of Shannon_H and Simpson_1-D values were found to be 1.78-1.9 and 0.79-0.85, respectively. These insights are important to understanding soil-plant-microorganism interactions in promoting sustainable agricultural practices in the Abras de Mantequilla wetland.
Citas
Alvarez-Mieles, G., Irvine, K., Griensven, A. V., Arias-Hidalgo, M., Torres, A., & Mynett, A. E. (2013). Relationships between aquatic biotic communities and water quality in a tropical river-wetland system (Ecuador). Environmental Science and Policy, 34, 115–127. https://doi.org/10.1016/j.envsci.2013.01.011
Balieiro, F. D. C., Franco, A. A., Fontes, R. L. F., Dias, L. E., Campello, E. F. C., & Faria, S. M. D. (2007). Evaluation of the throughfall and stemflow nutrient contents in mixed and pure plantations of Acacia mangium, Pseudosamenea guachapele and Eucalyptus grandis. Revista Árvore, 31, 339–346. https://doi.org/10.1590/S0100-67622007000200017
Bennett, A. E., & Classen, A.T. (2020). Climate change influences mycorrhizal fungal–plant interactions, but conclusions are limited by geographical study bias. Ecology, 101(4), e02978. https://doi.org/10.1002/ecy.2978
Bhupenchandra, I., Chongtham, S. K., Devi, A. G., Dutta, P., Sahoo, M. R., et al. (2024). Unlocking the potential of arbuscular mycorrhizal fungi: exploring role in plant growth promotion, nutrient uptake mechanisms, biotic stress alleviation, and sustaining agricultural production systems. Journal of Plant Growth Regulation, 1–39. In press. https://doi.org/10.1007/s00344-024-11467-9
Blair, B. C., & Perfecto, I. (2004). Successional status and root foraging for phosphorus in seven tropical tree species. Canadian Journal of Forest Research, 34(5), 1128–1135. https://doi.org/10.1139/x04-004
Blanchet, F., Legendre, P., & Borcard, D. (2008) Forward selection of explanatory variables. Ecology, 89(9), 2623–2632. https://doi.org/10.1890/07-0986.1
Blaszkowski, J. (2012). Glomeromycota. W. Szafer Institute of Botany, Polish Academy of Sciences. Krakow
Cardoso, I. M., & Kuyper, T. W. (2006). Mycorrhizas and tropical soil fertility. Agriculture, Ecosystems & Environment, 116(1-2), 72–84. https://doi.org/10.1016/j.agee.2006.03.011
Carteron, A., Vellend, M., & Laliberte, E. (2022). Mycorrhizal dominance reduces local tree species diversity across US forests. Nature Ecology & Evolution, 6(4), 370–374. https://doi.org/10.1038/s41559-021-01634-6
Chakraborty, S. K., Sanyal, P., & Ray, R. (2023). Diversity and classification of wetlands in international and national perspectives. In Wetlands Ecology: Eco-biological uniqueness of a Ramsar site (East Kolkata Wetlands, India) (pp. 167-226). Cham: Springer International Publishing. https://doi.org/10.1007/978-3-031-09253-4_3
Deng, M., Hu, S., Guo, L., Jiang, L., Huang, Y., Schmid, B., et al. (2023). Tree mycorrhizal association types control biodiversity-productivity relationship in a subtropical forest. Science Advances, 9(3), eadd4468. https://doi.org/10.1126/sciadv.add4468
Dray, S., Legendre, P., Blanchet, G. (2016). Packfor: forward selection with permutation. R package version 0.0-8/r136. https://R-Forge.Rproject.org/projects/sedar/ accessed November 1, 2023
Epihov, D. Z., Saltonstall, K., Batterman, S. A., Hedin, L. O., Hall, J. S., van Breugel, M., et al. (2021). Legume–microbiome interactions unlock mineral nutrients in regrowing tropical forests. Proceedings of the National Academy of Sciences, 118(11), e2022241118. https://doi.org/10.1073/pnas.2022241118
Faghihinia, M., Zou, Y., Chen, Z., Bai, Y., Li, W., Marrs, R., & Staddon, P. L. (2020). Environmental drivers of grazing effects on arbuscular mycorrhizal fungi in grasslands. Applied Soil Ecology, 153, 103591. https://doi.org/10.1016/j.apsoil.2020.103591
Gerdemann, J., & Nicolson, T. (1963). Spores of mycorrhizal endogone species extracted from soil by wet sieving and decanting. Transactions of the British Mycological Society, 46(2), 235–244. https://doi.org/10.1016/S0007-1536(63)80079-0
Ghosh, S., Anju, P., Pattanayak, R., & Sahu, N. C. (2024). Fisheries and Aquaculture in Wetland Ecosystems: A Review of Benefits, Risks, and Future Prospects in India. Journal of Coastal Research, 40(3), 598–612. https://doi.org/10.2112/JCOASTRES-D-23-00045.1
Guzmán-González, S., & Farías-Larios, J. (2005). Biología y regulación molecular de la micorriza arbuscular. Avances en Investigación Agropecuaria, 9(2), 17–31.
Hartmann, M., & Six, J. (2023). Soil structure and microbiome functions in agroecosystems. Nature Reviews Earth & Environment, 4(1), 4–18. https://doi.org/10.1038/s43017-022-00366-w
Hill, M., & Gauch, H. (1980). Detrended correspondence analysis: An improved ordination technique. Vegetatio, 42, 47–58. https://doi.org/10.1007/BF00048870
Huey, C. J., Gopinath, S. C., Uda, M. N. A., Zulhaimi, H. I., Jaafar, M. N., Kasim, F. H., & Yaakub, A. R. W. (2020). Mycorrhiza: a natural resource assists plant growth under varied soil conditions. 3 Biotech, 10(5), 204. https://doi.org/10.1007/s13205-020-02188-3
Irvine, K., Dickens, C., Castello, L., Bredin, I., & Finlayson, C. (2022). Vegetated wetlands: from ecology to conservation management. Fundamentals of Tropical Freshwater Wetlands, 1, 589-639. https://doi.org/10.1016/B978-0-12-822362-8.00023-2
Islam, M., Al-Hashimi, A., Ayshasiddeka, M., Ali, H., & El Enshasy, H. (2022). Prevalence of mycorrhizae in host plants and rhizosphere soil: A biodiversity aspect. PloS One, 17(8), e0273463. https://doi.org/10.1371/journal.pone.0273463
Janowski, D., & Leski, T. (2022). Factors in the distribution of mycorrhizal and soil fungi. Diversity, 14(12), 1122. https://doi.org/10.3390/d14121122
Jerbi, M., Labidi, S., Lounes-Hadj Sahraoui, A., Chaar, H., & Ben Jeddi, F. (2020). Higher temperatures and lower annual rainfall do not restrict, directly or indirectly, the mycorrhizal colonization of barley (Hordeum vulgare L.) under rainfed conditions. PloS One, 15(11), e0241794. https://doi.org/10.1371/journal.pone.0241794
Kupka, D., & Gruba, P. (2022). Effect of pH on the sorption of dissolved organic carbon derived from six tree species in forest soils. Ecological Indicators, 140, 108975. https://doi.org/10.1016/j.ecolind.2022.108975
Lambers, H., Wright, I. J., Guilherme Pereira, C., Bellingham, P. J., Bentley, L. P., Boonman, A., et al. (2021). Leaf manganese concentrations as a tool to assess belowground plant functioning in phosphorus-impoverished environments. Plant and soil, 461, 43–61. https://doi.org/10.1007/s11104-020-04690-2
Liu, M., Shen, Y., Li, Q., Xiao, W., & Song, X. (2021). Arbuscular mycorrhizal fungal colonization and soil pH induced by nitrogen and phosphorus additions affects leaf C: N: P stoichiometry in Chinese fir (Cunninghamia lanceolata) forests. Plant and Soil, 461, 421–440. https://doi.org/10.1007/s11104-021-04831-1
Luo, Y. H., Ma, L. L., Seibold, S., Cadotte, M. W., Burgess, K. S., Tan, S. L., et al. (2023). The diversity of mycorrhiza‐associated fungi and trees shapes subtropical mountain forest ecosystem functioning. Journal of Biogeography, 50(4), 715–729. https://doi.org/10.1111/jbi.14563
Ma, X., Geng, Q., Zhang, H., Bian, C., Chen, HY, Jiang, D., & Xu, X. (2021). Global negative effects of nutrient enrichment on arbuscular mycorrhizal fungi, plant diversity and ecosystem multifunctionality. New Phytologist, 229(5), 2957–2969. https://doi.org/10.1111/nph.17077
MAE. (2022). Classification system of ecosystems of continental Ecuador. Undersecretariat of Natural Heritage, Ministry of the Environment of Ecuador, Quito.
Oksanen, J., Blanchet, G., Kindt, R., Legendre, P., Minchin, P., O'Hara, R., Simpson, G., Solymos, P., Stevens, M., & Wagner, H. (2015). Vegan: Community Ecology Package. R package version 2. 3-0. http://CRAN.R-project.org/package=vegan accessed November 4, 2023
Painii-Montero, V. F., Santillán-Muñoz, O., Barcos-Arias, M., Portalanza, D., Durigon, A., & Garcés-Fiallos, F. R. (2020). Towards indicators of sustainable development for soybeans productive units: a multicriteria perspective for the Ecuadorian coast. Ecological Indicators, 119, 106800. https://doi.org/10.1016/j.ecolind.2023.111405
Portalanza, D., Torres-Ulloa, M., Arias-Hidalgo, M., Piza, C., Villa-Cox, G., Garcés-Fiallos, F. R., Álava, E., Durigon, A., & Espinel, R. (2024). Ecosystem services valuation in the Abras de Mantequilla wetland system: A comprehensive analysis. Ecological Indicators, 158, 111405. https://doi.org/10.1016/j.ecolind.2023.111405
Quevedo, O. (2008). Ramsar File for the Abras de Mantequilla Wetland - Ecuador 2008. Guayaquil, Ecuador. Retrieved from http://suia.ambiente.gob.ec
Qiu, L., Bi, Y., jiang, B., Wang, Z., Zhang, Y., & Zhakypbek, Y. (2019). Arbuscular mycorrhizal fungi ameliorate the chemical properties and enzyme activities of rhizosphere soil in reclaimed mining subsidence in northwestern China. Journal of Arid Land, 11, 135–147. https://doi.org/10.1007/s40333-018-0019-9
R Core Team, (2015). A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna.
Redecker, D., Schüßler, A., Stockinger, H., Stürmer, S., Morton, J., Walker, C. (2013). An evidence-based consensus for the classification of arbuscular mycorrhizal fungi (Glomeromycota). Mycorrhiza, 23(7), 515–531. https://doi.org/10.1007/s00572-013-0486-y
Rillig, M. C. (2004). Arbuscular mycorrhizae, glomalin, and soil aggregation. Canadian Journal of Soil Science, 84(4), 355–363. https://doi.org/10.4141/S04-003
Rożek, K., Rola, K., Błaszkowski, J., Leski, T., & Zubek, S. (2020). How do monocultures of fourteen forest tree species affect arbuscular mycorrhizal fungi abundance and species richness and composition in soil. Forest Ecology and Management, 465, 118091. https://doi.org/10.1016/j.foreco.2020.118091
Shi, J., Wang, X., & Wang, E. (2023). Mycorrhizal symbiosis in plant growth and stress adaptation: from genes to ecosystems. Annual Review of Plant Biology, 74(1), 569–607. https://doi.org/10.1146/annurev-arplant-061722-090342
Singavarapu, B., Beugnon, R., Bruelheide, H., Cesarz, S., Du, J., Eisenhauer, N., et al. (2022). Tree mycorrhizal type and tree diversity shape the forest soil microbiota. Environmental Microbiology, 24(9), 4236–4255. https://doi.org/10.1111/1462-2920.15690
Singh, D., Sillu, D., Kumar, A., & Agnihotri, S. (2021). Dual nanozyme characteristics of iron oxide nanoparticles alleviate salinity stress and promote the growth of an agroforestry tree, Eucalyptus tereticornis Sm. Environmental Science: Nano, 8(5), 1308–1325. https://doi.org/10.1039/D1EN00040C
Solís-Rodríguez, U. R., Ramos-Zapata, J.A., Ramos-Zapata, J. A., Hernández-Cuevas, L., & Salinas- Peba, L. (2020). Arbuscular mycorrhizal fungi diversity and distribution in tropical low flooding forests in Mexico. Mycological Progress, 19, 195–204. https://doi.org/10.1007/s11557-019-01550-x
Tedersoo, L., Bahram, M., & Zobel, M. (2020). How mycorrhizal associations drive plant population and community biology. Science, 367(6480), eaba1223. https://doi.org/10.1126/science.aba1223
Teixeira, P., Donagemma, G., Fontana, A., Teixeira, W. (2017). Manual de métodos de análise de solo. Embrapa Solos, Brasilia. 574 p.
Ullah, A., Gao, D., & Wu, F. (2024). Common mycorrhizal network: the predominant socialist and capitalist responses of possible plant–plant and plant–microbe interactions for sustainable agriculture. Frontiers in Microbiology, 15, 1183024. https://doi.org/10.3389/fmicb.2024.1183024
Usman, M., Ho- Plágaro, T., Frank, H., Calvo-Polanco, M., Gaillard, I., Garcia, K., & Zimmermann, D. (2021). Mycorrhizal symbiosis for better adaptation of trees to abiotic stress caused by climate change in temperate and boreal forests. Frontiers in Forests and Global Change, 4, 742392 https://doi.org/10.3389/ffgc.2021.742392
Vieira, L. C., Silva, D. K., Escobar, I. E., Silva, J. M., Moura, I. A., Oehl, F., & Silva, G. A. (2020). Changes in an arbuscular mycorrhizal fungi community along an environmental gradient. Plants, 9(1), 52. https://doi.org/10.3390/plants9010052
Wang, J., Wang, G. G., Zhang, B., Yuan, Z., & Fu, Z. (2019). Arbuscular mycorrhizal fungi associated with tree species in a planted forest of Eastern China. Forests, 10(5), 424. https://doi.org/10.3390/f10050424
Waring, B. G., Gei, M. G., Rosenthal, L., & Powers, J. S. (2016). Plant–microbe interactions along a gradient of soil fertility in tropical dry forest. Journal of Tropical Ecology, 32(4), 314–323. https://doi.org/10.1017/S0266467416000286
Zhang, J., Quan, C., Ma, L., Chu, G., Liu, Z., & Tang, X. (2021). Plant community and soil properties drive arbuscular mycorrhizal fungal diversity: A case study in tropical forests. Soil Ecology Letters, 3, 52–62. https://doi.org/10.1007/s42832-020-0049-z
Zhen, L. I., Songlin, W. U., Yunjia, L. I. U., Qing, Y. I., Merinda, H. A. L. L., Narottam, S. A. H. A., et al. (2024). Arbuscular mycorrhizal fungi regulate plant mineral nutrient uptake and partitioning in iron ore tailings undergoing eco-engineered pedogenesis. Pedosphere, 34(2), 385–398. https://doi.org/10.1016/j.pedsph.2023.04.004
Descargas
Publicado
Cómo citar
Número
Sección
Licencia
Derechos de autor 2024 Scientia Agropecuaria

Esta obra está bajo una licencia internacional Creative Commons Atribución-NoComercial 4.0.
Los autores que publican en esta revista aceptan los siguientes términos:
a. Los autores conservan los derechos de autor y conceden a la revista el derecho publicación, simultáneamente licenciada bajo una licencia de Creative Commons que permite a otros compartir el trabajo, pero citando la publicación inicial en esta revista.
b. Los autores pueden celebrar acuerdos contractuales adicionales separados para la distribución no exclusiva de la versión publicada de la obra de la revista (por ejemplo, publicarla en un repositorio institucional o publicarla en un libro), pero citando la publicación inicial en esta revista.
c. Se permite y anima a los autores a publicar su trabajo en línea (por ejemplo, en repositorios institucionales o en su sitio web) antes y durante el proceso de presentación, ya que puede conducir a intercambios productivos, así como una mayor citación del trabajo publicado (ver efecto del acceso abierto).




