Dinámica de crecimiento, esporulación y captura de hongos asexuales sobre Meloidogyne sp., in vitro
DOI:
https://doi.org/10.17268/sci.agropecu.2024.009Palabras clave:
Agricultura tropical, captura de nematodos, control biológico, in vitro, OrbiliaceaeResumen
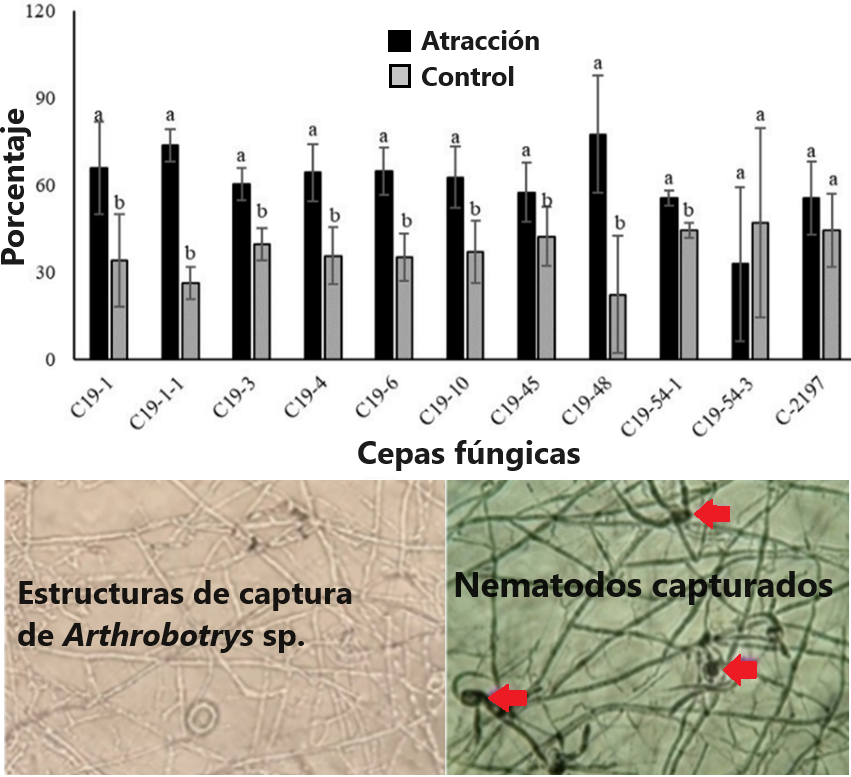
Los nematodos formadores de agallas son perjudiciales para los cultivos agrícolas. El objetivo de esta investigación fue evaluar el crecimiento, esporulación y captura de hongos asexuales contra Meloidogyne sp. en condiciones in vitro. La identificación molecular de los hongos asexuales se realizó con PCR utilizando secuencias de la región ITS del ADNr. Se evaluó el crecimiento y esporulación de Arthrobotrys, Dactylellina y Dactylaria en cinco medios de cultivos y dos tipos de substratos (cascarilla de arroz y maíz molido). Para la evaluación de atracción y captura de los nematodos se emplearon juveniles de segunda etapa de Meloidogyne sp. Todos los aislamientos fúngicos evaluados crecieron y esporularon en los medios de cultivos y substratos. Los hongos Arthrobotrys sp. (C19-1-1) y Dactylellina sp. (C19-48) mostraron mayor efectividad en la atracción y captura de Meloidogyne sp. Los aislamientos fúngicos evaluados tienen el potencial de colonizar diferentes medios de cultivos y substratos. Además, pueden formar hifas modificadas y especializadas que controlan nematodos juveniles de Meloidogyne sp. Por lo tanto, los futuros estudios se deberían centrar en evaluar los aislados fúngicos contra los nematodos formadores de agallas en condiciones de campo.
Citas
Al-Hakeem, A. M., Al-Molla, S. A., & Al-Tarjuman, J. K. (2022). Nematophagous Fungi. Texas Journal of Agriculture and Biological Sciences, 5, 37-42.
Altschul, S. F., Madden, T. L., Schäffer, A. A., Zhang, J., Zhang, Z., Miller, W., & Lipman, D. J. (1997). Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Research, 25(17), 3389-3402. https://doi.org/10.1093/nar/25.17.3389
Barker, K. R., Townshend, J. L., Bird, G. W., Thomason, I. J., & Dickson, D. W. (1986). Determining nematode population responses to control agents. En K. D. Hickey (Ed.), Methods for evaluating pesticides for control of plant pathogens (pp. 283-299). American Phytopathology Society Press, St Paul.
Barron, G. L. (1977). The nematode-destroying fungi. – Topics in Mycobiology No. 1. University of Guelph, Ontario. Lancaster Press, Lancaster, Pennsylvania.
Calderón, J., Miño-Castro, G., Llumiquinga, P., Abbas, M., Gutiérrez-Gutiérrez, C., Segovia-Salcedo, M., & Proaño, K. (2022). Distribution of Meloidogyne spp. In agricultural crops of Ecuador: A literature review (1976–2021). CABI Reviews, 2022. https://doi.org/10.1079/cabireviews202217028
Castagnone-Sereno, P., Esparrago, G., Abad, P., Leroy, F., & Bongiovanni, M. (1995). Satellite DNA as a target for PCR-specific detection of the plant-parasitic nematode Meloidogyne hapla. Current Genetics, 28(6), 566-570. https://doi.org/10.1007/BF00518170
Castañeda-Ruiz, R. F., Heredia, G., Gusmão, L. F. P., & Li, D.-W. (2016). Fungal Diversity of Central and South America. En D.-W. Li (Ed.), Biology of Microfungi (pp. 197-217). Springer International Publishing. https://doi.org/10.1007/978-3-319-29137-6_9
Cenis, J. L. (1992). Rapid extraction of fungal DNA for PCR amplification. Nucleic Acids Research, 20(9), 2380. https://doi.org/10.1093/nar/20.9.2380
Chávez-Arteaga, K. T., Cedeño-Moreira, Á. V., Canchignia-Martínez, H. F., & Garcés-Fiallos, F. R. (2022). Candidate rhizobacteria as plant growth-promoters and root-knot nematode controllers in tomato plants. Scientia Agropecuaria, 13(4), 423-432. https://doi.org/10.17268/sci.agropecu.2022.038
Cooke, R. C., & Godfrey, B. E. S. (1964). A key to the nematode-destroying fungi. Transactions of the British Mycological Society, 47(1), 61-74. https://doi.org/10.1016/S0007-1536(64)80081-4
da Silva, M. E., de Araújo, J. V., Braga, F. R., Borges, L. A., Soares, F. E. F., dos Santos Lima, W., & Guimarães, M. P. (2013). Mycelial mass production of fungi Duddingtonia flagrans and Monacrosporium thaumasium under different culture conditions. BMC Research Notes, 6(1), 340. https://doi.org/10.1186/1756-0500-6-340
Dávila, L., & Hío, J. C. (2005). Evaluación de la actividad biocontroladora de Arthrobotrys sp. Y Paecilomyces sp. Sobre Meloidogyne javanica in vitro y bajo condiciones de invernadero en Crisantemo (Drendranthema grandiflora Andernson). Agronomía Colombiana, 23(1), 91-101.
de Hollanda, T., Monteiro, T. S. A., Braga, F. R., de Freitas Soares, F. E., de Mello, I. N. K., et al. (2016). Assessment of compatibility between the nematophagous fungi Arthrobotrys robusta and Duddingtonia flagrans under laboratory conditions. Revista Iberoamericana De Micologia, 33(2), 129-130. https://doi.org/10.1016/j.riam.2015.07.001
Delgado, E. F., Valdez, A. T., Covarrubias, S. A., Tosi, S., & Nicola, L. (2021). Soil fungal diversity of the Aguarongo Andean Forest (Ecuador). Biology, 10(12), 1289. https://doi.org/10.3390/biology10121289
Drechsler, C. (1937). Some Hyphomycetes that prey on free-living terricolous nematodes. Mycologia, 29(4), 447-552. https://doi.org/10.1080/00275514.1937.12017222
Duddington, C. L. (1955). Notes on the technique of handling predacious fungi. Transactions of the British Mycological Society, 38(2), 97-103. https://doi.org/10.1016/S0007-1536(55)80021-6
Elling, A. A. (2013). Major emerging problems with minor Meloidogyne species. Phytopathology, 103(11), 1092-1102. https://doi.org/10.1094/PHYTO-01-13-0019-RVW
Elshafie, A., Al-Mueini, R., Al-Bahry, S., Akindi, A., Mahmoud, I., & Al-Rawahi, S. (2006). Diversity and trapping efficiency of nematophagous fungi from Oman. Phytopathologia mediterranea, 45, 266-270. https://doi.org/10.1400/56490
Flores, B. G., Ponce, I. M., Plascencia Espinosa, M. Á., Mendieta Moctezuma, A., & López y López, V. E. (2021). Advances in the biological control of phytoparasitic nematodes via the use of nematophagous fungi. World Journal of Microbiology and Biotechnology, 37(10), 180. https://doi.org/10.1007/s11274-021-03151-x
Forghani, F., & Hajihassani, A. (2020). Recent advances in the development of environmentally benign treatments to control root-knot nematodes. Frontiers in Plant Science, 11. https://www.frontiersin.org/articles/10.3389/fpls.2020.01125
Hsueh, Y.-P., Gronquist, M. R., Schwarz, E. M., Nath, R. D., Lee, C.-H., Gharib, S., Schroeder, F. C., & Sternberg, P. W. (2017). Nematophagous fungus Arthrobotrys oligospora mimics olfactory cues of sex and food to lure its nematode prey. eLife, 6, e20023. https://doi.org/10.7554/eLife.20023
Hussain, M., Maňasová, M., Zouhar, M., & Ryšánek, P. (2020). Comparative virulence assessment of different nematophagous fungi and chemicals against northern root-knot nematodes, Meloidogyne hapla, on carrots. Pakistan J. Zool, 52(1), 199-206. https://doi.org/10.17582/journal.pjz/2020.52.1.199.206
Hussain, M., Zouhar, M., & Ryšánek, P. (2018). Attraction of root knot nematodes, Meloidogyne incognita, to living mycelium of nematophagous fungi. Pakistan Journal of Zoology, 50(6), 2073-2078. https://doi.org/10.17582/journal.pjz/2018.50.6.2073.2078
Jiang, X., Xiang, M., & Liu, X. (2017). Nematode-trapping fungi. Microbiology Spectrum, 5(1), 5.1.10. https://doi.org/10.1128/microbiolspec.FUNK-0022-2016
Karakaş, M. (2020). Nematode-destroying fungi: Infection structures, interaction mechanisms and biocontrol. Communications Faculty of Sciences University of Ankara Series C Biology, 29(1), Article 1.
Kassam, R., Yadav, J., Chawla, G., Kundu, A., Hada, A., Jaiswal, N., Bollinedi, H., Kamil, D., Devi, P., & Rao, U. (2021). Identification, characterization, and evaluation of nematophagous fungal species of Arthrobotrys and Tolypocladium for the management of Meloidogyne incognita. Frontiers in Microbiology, 12, 790223.
Khan, A., Ahmad, G., Haris, M., & Khan, A. A. (2023). Bio-organics management: Novel strategies to manage root-knot nematode, Meloidogyne incognita pest of vegetable crops. Gesunde Pflanzen, 75(1), 193-209. https://doi.org/10.1007/s10343-022-00679-2
Kumar, D., Maurya, N., Kumar, P., Singh, H., & Addy, S. K. (2015). Assessment of germination and carnivorous activities of a nematode-trapping fungus Arthrobotrys dactyloides in fungistatic and fungicidal soil environment. Biological Control, 82, 76-85. https://doi.org/10.1016/j.biocontrol.2014.12.014
Li, J., Hyde, K., & Zhang, K.-Q. (2014). Methodology for studying nematophagous fungi. En K.-Q. Zhang & K. D. Hyde (Eds.), Nematode-Trapping Fungi (pp. 13-40). Springer Netherlands. https://doi.org/10.1007/978-94-017-8730-7_2
Lin, H.-C., & Hsueh, Y.-P. (2021). Laboratory maintenance and culturing of the nematode-trapping fungus Arthrobotrys oligospora. Current Protocols, 1(2), e41. https://doi.org/10.1002/cpz1.41
Liu, Q., Li, D., Jiang, K., Zhang, K.-Q., & Yang, J. (2022). AoPEX1 and AoPEX6 are required for mycelial growth, conidiation, stress response, fatty acid utilization, and trap formation in Arthrobotrys oligospora. Microbiology Spectrum, 10(2), e00275-22. https://doi.org/10.1128/spectrum.00275-22
Magan. (2007). Fungi in extreme environments. En C. P. Kubicek & I. S. Druzhinina (Eds.), Environmental and Microbial Relationships (pp. 85-103). Springer. https://doi.org/10.1007/978-3-540-71840-6_6
Martins, P. L., Nozaki, M. de H., Barbosa, B. F. F., dos Santos, J. M., & Barbosa, J. C. (2009). Crescimento e esporulação de duas espécies de Arthrobotrys corda em diferentes meios de cultura e dois ambientes. Bioscience Journal, 25(2). http://acervodigital.unesp.br/handle/11449/1400
Matsushima, T. (1971). Microfungi of the Solomon Islands and Papua-New Guinea. Kobe, Japan.
Matsushima, T. (1975). Icones microfungorum a Matsushima lectorum. Nippon Printing & Publishing Co., Osaka, Japan. p. 209.
Moosavi, M. R., & Zare, R. (2012). Fungi as biological control agents of plant-parasitic nematodes. En J. M. Mérillon & K. G. Ramawat (Eds.), Plant Defence: Biological Control (pp. 67-107). Springer Netherlands. https://doi.org/10.1007/978-94-007-1933-0_4
Mukhtar, T., Hussain, M. A., Kayani, M. Z., & Aslam, M. N. (2014). Evaluation of resistance to root-knot nematode (Meloidogyne incognita) in okra cultivars. Crop Protection, 56, 25-30. https://doi.org/10.1016/j.cropro.2013.10.019
Nagaraj, G., Kannan, R., Raguchander, T., Narayanan, S., & Saravanakumar, D. (2024). Nematicidal action of Clonostachys rosea against Meloidogyne incognita: In-vitro and in-silico analyses. Journal of Taibah University for Science, 18(1), 2288723. https://doi.org/10.1080/16583655.2023.2288723
Nicola, L., Tosi, S., & Savini, D. (2014). In vitro evaluation of nematophagous activity of fungal isolates. Journal of Basic Microbiology, 54(1), 1-5. https://doi.org/10.1002/jobm.201200431
Orozco, M., Jiménez, A., Acuña, O., & Álvarez, V. (2015). Determinación del crecimiento de hongos nematófagos en diversas fuentes de carbono. Agronomía Costarricense, 39(2), 143-152.
Quevedo, A., Vera-Morales, M., Espinoza-Lozano, F., Castañeda-Ruiz, R. F., Sosa del Castillo, D., & Magdama, F. (2021). Assessing the predatory activity of Arthrobotrys oligosporus strain C-2197 as biocontrol of the root-knot nematode Meloidogyne spp. Bionatura, 6(1), 1586-1592. https://doi.org/10.21931/RB/2021.06.01.22
Rani, L., Thapa, K., Kanojia, N., Sharma, N., Singh, S., Grewal, A. S., Srivastav, A. L., & Kaushal, J. (2021). An extensive review on the consequences of chemical pesticides on human health and environment. Journal of Cleaner Production, 283, 124657. https://doi.org/10.1016/j.jclepro.2020.124657
Ritchie, B. J. (2001). Mycological media and methods. Plant pathologist’s pocketbook, 410-431. https://doi.org/10.1079/9780851994581.0410
Rubner, A. (1994). Predaceous fungi from Ecuador. Mycotaxon, 51, 143-151.
Rubner, A. (1996). Revision of predacious hyphomycetes in the Dactylella—Monacrosporium complex. Studies in Mycology 39: 134 p.
Saha, T., & Khan, M. (2016). Evaluation of bioformulations for management of root knot nematode (Meloidogyne incognita) infecting tuberose. Pakistan Journal of Zoology, 48(3), 651-656.
Saxena, G., Dayal, R., & Mukerji, K. G. (1987). Interaction of nematodes with nematophagus fungi: Induction of trap formation, attraction and detection of attractants. FEMS Microbiology Ecology, 3(6), 319-327. https://doi.org/10.1111/j.1574-6968.1987.tb02408.x
Shirazi, R., Fatemy, N., & Naeimi, S. (2019). Solid-state fermentation and viability of Pochonia chlamydosporia and Purpureocillium lilacinum on some organic substrates. BioControl in Plant Protection, 6(2), 1-14. https://doi.org/10.22092/bcpp.2019.119398
Siddiqui, Z. A., & Aziz, S. (2024). Plant parasitic nematode-fungus interactions: Recent concepts and mechanisms. Plant Physiology Reports. https://doi.org/10.1007/s40502-023-00762-4
Silva, J. C. P., Nunes, T. C. S., Guimarães, R. A., Pylro, V. S., Costa, L. S. A. S., Zaia, R., Campos, V. P., & Medeiros, F. H. V. (2022). Organic practices intensify the microbiome assembly and suppress root-knot nematodes. Journal of Pest Science, 95(2), 709-721. https://doi.org/10.1007/s10340-021-01417-9
Soares, F. E. de F., Sufiate, B. L., & de Queiroz, J. H. (2018). Nematophagous fungi: Far beyond the endoparasite, predator and ovicidal groups. Agriculture and Natural Resources, 52(1), 1-8. https://doi.org/10.1016/j.anres.2018.05.010
Soliman, M. S., El-Deriny, M. M., Ibrahim, D. S., Zakaria, H., & Ahmed, Y. (2021). Suppression of root-knot nematode Meloidogyne incognita on tomato plants using the nematode trapping fungus Arthrobotrys oligospora Fresenius. Journal of Applied Microbiology, 131(5), 2402-2415. https://doi.org/10.1111/jam.15101
Su, H., Zhao, Y., Zhou, J., Feng, H., Jiang, D., Zhang, K.-Q., & Yang, J. (2017). Trapping devices of nematode-trapping fungi: Formation, evolution, and genomic perspectives. Biological Reviews, 92(1), 357-368. https://doi.org/10.1111/brv.12233
Swe, A., Jeewon, R., Pointing, S. B., & Hyde, K. D. (2009). Diversity and abundance of nematode-trapping fungi from decaying litter in terrestrial, freshwater and mangrove habitats. Biodiversity and Conservation, 18(6), 1695-1714. https://doi.org/10.1007/s10531-008-9553-7
Tapia-Vázquez, I., Montoya-Martínez, A. C., De los Santos-Villalobos, S., Ek-Ramos, M. J., Montesinos-Matías, R., & Martínez-Anaya, C. (2022). Root-knot nematodes (Meloidogyne spp.) a threat to agriculture in Mexico: Biology, current control strategies, and perspectives. World Journal of Microbiology and Biotechnology, 38(2), 26. https://doi.org/10.1007/s11274-021-03211-2
Tazi, H., Hamza, M. A., Hallouti, A., Benjlil, H., Idhmida, A., et al. (2021). Biocontrol potential of nematophagous fungi against Meloidogyne spp. Infecting tomato. Organic Agriculture, 11(1), 63-71. https://doi.org/10.1007/s13165-020-00325-z
Tigano, M. S., Carneiro, R. M. D. G., Jeyaprakash, A., Dickson, D. W., & Adams, B. J. (2005). Phylogeny of Meloidogyne spp. Based on 18S rDNA and the intergenic region of mitochondrial DNA sequences. Nematology, 7(6), 851-862. https://doi.org/10.1163/156854105776186325
Torto, B., Cortada, L., Murungi, L. K., Haukeland, S., & Coyne, D. L. (2018). Management of cyst and root knot nematodes: A chemical ecology perspective. Journal of Agricultural and Food Chemistry, 66(33), 8672-8678. https://doi.org/10.1021/acs.jafc.8b01940
Tsiafouli, M. A., Thébault, E., Sgardelis, S. P., de Ruiter, P. C., van der Putten, W. H., et al. (2015). Intensive agriculture reduces soil biodiversity across Europe. Global Change Biology, 21(2), 973-985. https://doi.org/10.1111/gcb.12752
Vera-Morales, M., Castañeda-Ruiz, R. F., Sosa, D., Quevedo, A., Naranjo-Morán, J., Serrano, L., & Ratti, M. F. (2022). Mecanismos de captura, colonización y alimentación empleados por parásitos y predadores de nematodos. Ecosistemas, 31(3), Article 3. https://doi.org/10.7818/ECOS.2390
Vera-Morales, M., López Medina, S. E., Naranjo-Morán, J., Quevedo, A., & Ratti, M. F. (2023). Nematophagous fungi: A review of their phosphorus solubilization potential. Microorganisms, 11(1), Article 1. https://doi.org/10.3390/microorganisms11010137
Vidal-Diez de Ulzurrun, G., & Hsueh, Y.-P. (2018). Predator-prey interactions of nematode-trapping fungi and nematodes: Both sides of the coin. Applied Microbiology and Biotechnology, 102(9), 3939-3949. https://doi.org/10.1007/s00253-018-8897-5
Wang, D., Ma, N., Rao, W., & Zhang, Y. (2023). Recent advances in life history transition with netmatode-trapping fungus Arthrobotrys oligospora and its application in sustainable agriculture. Pathogens (Basel, Switzerland), 12(3), 367. https://doi.org/10.3390/pathogens12030367
Wang, F.-H., Xu, Q., Wang, B., Wang, K.-Y., Xue, Y.-J., et al. (2017). Isolation, identification and characterisation of the nematophagous fungus Arthrobotrys thaumasia (Monacrosporium thaumasium) from China. Biocontrol Science and Technology, 27(3), 378-392. https://doi.org/10.1080/09583157.2017.1291908
Wang, J., Huang, X., Niu, S., Hu, Z., Li, H., et al. (2019). Thioredoxin1 regulates conidia formation, hyphal growth, and trap formation in the nematode-trapping fungus Arthrobotrys oligospora. Annals of Microbiology, 69(12), Article 12. https://doi.org/10.1007/s13213-019-01511-5
White, T. J., Bruns, T., Lee, S., & Taylor, J. (1990). Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. En M. A. Innis, D. H. Gelfand, J. J. Sninsky, & T. J. White (Eds.), PCR Protocols (pp. 315-322). Academic Press. https://doi.org/10.1016/B978-0-12-372180-8.50042-1
Yang, C.-T., Vidal-Diez de Ulzurrun, G., Gonçalves, A. P., Lin, H.-C., Chang, C.-W., et al. (2020). Natural diversity in the predatory behavior facilitates the establishment of a robust model strain for nematode-trapping fungi. Proceedings of the National Academy of Sciences, 117(12), 6762-6770. https://doi.org/10.1073/pnas.1919726117
Yu, Z., Mo, M., Zhang, Y., & Zhang, K.-Q. (2014). Taxonomy of nematode-trapping fungi from Orbiliaceae, Ascomycota. En K.-Q. Zhang & K. D. Hyde (Eds.), Nematode-Trapping Fungi (pp. 41-210). Springer Netherlands. https://doi.org/10.1007/978-94-017-8730-7_3
Zhang, K.-Q., & Hyde, K. D. (Eds.). (2014). Nematode-Trapping Fungi. Fungal Diversity Research Series, 23, Springer. 403 p.
Publicado
Cómo citar
Número
Sección
Licencia
Derechos de autor 2024 Scientia Agropecuaria

Esta obra está bajo una licencia internacional Creative Commons Atribución-NoComercial 4.0.
Los autores que publican en esta revista aceptan los siguientes términos:
a. Los autores conservan los derechos de autor y conceden a la revista el derecho publicación, simultáneamente licenciada bajo una licencia de Creative Commons que permite a otros compartir el trabajo, pero citando la publicación inicial en esta revista.
b. Los autores pueden celebrar acuerdos contractuales adicionales separados para la distribución no exclusiva de la versión publicada de la obra de la revista (por ejemplo, publicarla en un repositorio institucional o publicarla en un libro), pero citando la publicación inicial en esta revista.
c. Se permite y anima a los autores a publicar su trabajo en línea (por ejemplo, en repositorios institucionales o en su sitio web) antes y durante el proceso de presentación, ya que puede conducir a intercambios productivos, así como una mayor citación del trabajo publicado (ver efecto del acceso abierto).




