Compuestos bioactivos de bacterias y hongos en el control de nematodos fitopatógenos: mecanismos de acción, interacciones y aplicaciones
DOI:
https://doi.org/10.17268/sci.agropecu.2024.011Palabras clave:
Bacterias, Biocontrol, Compuestos químicos, Hongos, Nematodos fitopatógenosResumen
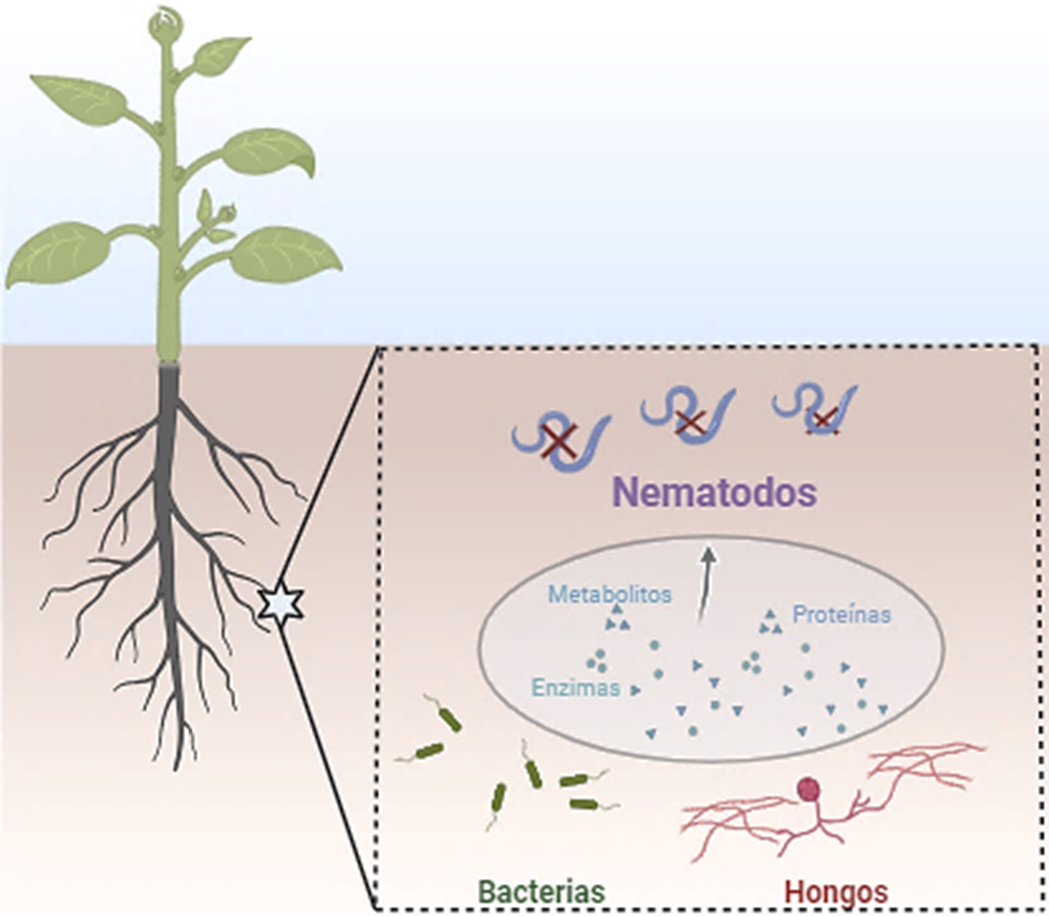
Los nematodos fitopatógenos son una amenaza para la producción agrícola, causando enfermedades en las plantas y pérdidas económicas en todo el mundo. En la actualidad, la búsqueda de compuestos de origen biológico para estos nematodos, como una alternativa amigable con el ambiente, se ha convertido en prioridad. Los compuestos derivados de microorganismos cuentan con diferentes mecanismos de acción para reducir las poblaciones de juveniles infecciosos de nematodos. A pesar de que la literatura reporta revisiones sobre los microorganismos y sus metabolitos nematicidas, no se destacan las interacciones entre hongos, bacterias y nematodos, así como de los compuestos que se generan en tales interacciones. El objetivo de esta revisión fue discutir los avances recientes sobre compuestos químicos secretados por microorganismos y mecanismos de interacción para el control biológico de nematodos fitopatógenos. Los resultados se organizaron en función de i) mecanismos de acción, ii) proceso de infección, iii) compuestos nematicidas, iv) interacciones y aplicaciones a las soluciones en campo y v) desafíos actuales. El enfoque de esta revisión contribuye a una mejor comprensión de la utilidad de los compuestos producidos por bacterias y hongos que permitan incorporarlos en el desarrollo de planes de manejo para el control de nematodos.
Citas
Abd El-Rahman, A. F., Shaheen, H. A., Abd El-Aziz, R. M., & Ibrahim, D. S. S. (2019). Influence of hydrogen cyanide-producing rhizobacteria in controlling the crown gall and root-knot nematode, Meloidogyne incognita. Egyptian Journal of Biological Pest Control, 29(1), 1–11. https://doi.org/10.1186/S41938-019-0143-7/TABLES/8
Anderson, A. J., & Kim, Y. C. (2018). Biopesticides produced by plant-probiotic Pseudomonas chlororaphis isolates. Crop Protection, 105, 62–69. https://doi.org/10.1016/J.CROPRO.2017.11.009
Andersson, K. M., Meerupati, T., Levander, F., Friman, E., Ahrén, D., & Tunlida, A. (2013). Proteome of the nematode-trapping cells of the fungus Monacrosporium haptotylum. Applied and Environmental Microbiology, 79(16), 4993–5004. https://doi.org/10.1128/AEM.01390-13
Anitha, R., & Murugesan, K. (2005). Production of gliotoxin on natural substrates by Trichoderma virens. Journal of Basic Microbiology, 45(1), 12–19. https://doi.org/10.1002/JOBM.200410451
Antil, S., Kumar, R., Pathak, D. V., & Kumari, A. (2023). Recent advances in utilizing bacteria as biocontrol agents against plant parasitic nematodes emphasizing Meloidogyne spp. Biological Control, 183, 105244. https://doi.org/10.1016/J.BIOCONTROL.2023.105244
Araldi-Brondolo, S. J., Spraker, J., Shaffer, J. P., Woytenko, E. H., Baltrus, D. A., Gallery, R. E., & Arnold, A. E. (2017). Bacterial Endosymbionts: Master Modulators of Fungal Phenotypes. Microbiology Spectrum, 5(5). https://doi.org/10.1128/MICROBIOLSPEC.FUNK-0056-2016
Arendt, K. R., Hockett, K. L., Araldi-Brondolo, S. J., Baltrus, D. A., & Arnold, A. E. (2016). Isolation of Endohyphal Bacteria from Foliar Ascomycota and In Vitro Establishment of Their Symbiotic Associations. Applied and Environmental Microbiology, 82(10), 2943–2949. https://doi.org/10.1128/AEM.00452-16
Atif, A. M., Elzamik, F. I., Mohamed, G. M., Al-Quwaie, D. A., Ashkan, M. F., et al. (2023). Biological control of the root-knot nematode (Meloidogyne incognita) on eggplants with various chitinase-producing Streptomyces strains. European Journal of Plant Pathology, 167(3), 371–394. https://doi.org/10.1007/S10658-023-02718-8/FIGURES/1
Ayaz, M. , Li, C.-H. , Ali, Q., Zhao, W. , Chi, Y.-K. , et al. (2023). Bacterial and Fungal Biocontrol Agents for Plant Disease Protection: Journey from Lab to Field, Current Status, Challenges, and Global Perspectives. Molecules 2023, Vol. 28, Page 6735, 28(18), 6735. https://doi.org/10.3390/MOLECULES28186735
Ayaz, M., Ali, Q., Farzand, A., Khan, A. R., Ling, H., & Gao, X. (2021). Nematicidal volatiles from Bacillus atrophaeus GBSC56 promote growth and stimulate induced systemic resistance in tomato against Meloidogyne incognita. International Journal of Molecular Sciences, 22(9). https://doi.org/10.3390/IJMS22095049
Barros, B. H. R., da Silva, S. H., Marques, E. dos R., Rosa, J. C., Yatsuda, A. P., Roberts, D. W., & Braga, G. U. L. (2010). A proteomic approach to identifying proteins differentially expressed in conidia and mycelium of the entomopathogenic fungus Metarhizium acridum. Fungal Biology, 114(7), 572–579. https://doi.org/10.1016/J.FUNBIO.2010.04.007
Beck, J. J., Alborn, H. T., Block, A. K., Christensen, S. A., Hunter, C. T., et al. (2018). Interactions among plants, insects, and microbes: elucidation of inter-organismal chemical communications in agricultural ecology. Journal of Agricultural and Food Chemistry, 66(26), 6663–6674. https://doi.org/10.1021/ACS.JAFC.8B01763
Bhat, A. A., Shakeel, A., Waqar, S., Handoo, Z. A., & Khan, A. A. (2023). Microbes vs. Nematodes: Insights into Biocontrol through Antagonistic Organisms to Control Root-Knot Nematodes. Plants 2023, Vol. 12, Page 451, 12(3), 451. https://doi.org/10.3390/PLANTS12030451
Bogner, C. W., Kamdem, R. S. T., Sichtermann, G., Matthäus, C., Hölscher, D., et al. (2017). Bioactive secondary metabolites with multiple activities from a fungal endophyte. Microbial Biotechnology, 10(1), 175–188. https://doi.org/10.1111/1751-7915.12467
Bui, H. X., & Desaeger, J. A. (2021). Volatile compounds as potential bio-fumigants against plant-parasitic nematodes – a mini review. Journal of Nematology, 53, 1–12. https://doi.org/10.21307/JOFNEM-2021-014
Bui, H. X., Hadi, B. A. R., Oliva, R., & Schroeder, N. E. (2020). Beneficial bacterial volatile compounds for the control of root-knot nematode and bacterial leaf blight on rice. Crop Protection, 135, 104792. https://doi.org/10.1016/J.CROPRO.2019.04.016
Camacho, M., de los Santos, B., Vela, M. D., & Talavera, M. (2023). Use of Bacteria Isolated from Berry Rhizospheres as Biocontrol Agents for Charcoal Rot and Root-Knot Nematode Strawberry Diseases. Horticulturae 2023, Vol. 9, Page 346, 9(3), 346. https://doi.org/10.3390/HORTICULTURAE9030346
Cao, H., Jiao, Y., Yin, N., Li, Y., Ling, J., Mao, Z., Yang, Y., & Xie, B. (2019). Analysis of the activity and biological control efficacy of the Bacillus subtilis strain Bs-1 against Meloidogyne incognita. Crop Protection, 122, 125–135. https://doi.org/10.1016/J.CROPRO.2019.04.021
Chavarria-Quicaño, E., Contreras-Jácquez, V., Carrillo-Fasio, A., De la Torre-González, F., & Asaff-Torres, A. (2023). Native Bacillus paralicheniformis isolate as a potential agent for phytopathogenic nematodes control. Frontiers in Microbiology, 14, 1213306. https://doi.org/10.3389/FMICB.2023.1213306/BIBTEX
Chen, S. A., Lin, H. C., Schroeder, F. C., & Hsueh, Y. P. (2021). Prey sensing and response in a nematode-trapping fungus is governed by the MAPK pheromone response pathway. Genetics, 217(2). https://doi.org/10.1093/GENETICS/IYAA008
Chen, T.-H., Hsu, C.-S., Tsai, P.-J., Ho, Y.-F., & Lin, N.-S. (2001). Heterotrimeric G-protein and signal transduction in the nematode-trapping fungus Arthrobotrys dactyloides. Planta, 212(5), 858–863. https://doi.org/10.1007/s004250000451
Chen, W., Wang, J., Huang, D., Cheng, W., Shao, Z., Cai, M., Zheng, L., Yu, Z., & Zhang, J. (2022). Volatile organic compounds from Bacillus aryabhattai MCCC 1K02966 with multiple modes against Meloidogyne incognita. Molecules, 27(1), 103. https://doi.org/10.3390/MOLECULES27010103
Cheng, W., Yang, J., Nie, Q., Huang, D., Yu, C., et al. (2017). Volatile organic compounds from Paenibacillus polymyxa KM2501-1 control Meloidogyne incognita by multiple strategies. Scientific Reports, 7(1), 1–11. https://doi.org/10.1038/s41598-017-16631-8
Cheng, W., Yang, X., Zeng, L., Huang, D., Cai, M., Zheng, L., Yu, Z., & Zhang, J. (2020). Evaluation of multiple impacts of furfural acetone on nematodes in vitro and control efficiency against root-knot nematodes in pots and fields. Antibiotics, 9(9), 1–14. https://doi.org/10.3390/ANTIBIOTICS9090605
Cruz, D. G., Costa, L. M., Rocha, L. O., Retamal, C. A., Vieira, R. A. M., et al. (2015). Serine proteases activity is important for the interaction of nematophagous fungus Duddingtonia flagrans with infective larvae of trichostrongylides and free-living nematodes Panagrellus spp. Fungal Biology, 119(8), 672–678. https://doi.org/10.1016/J.FUNBIO.2015.03.005
Dackman, C., & Nordbring-Hertz, B. (1992). Conidial traps — a new survival structure of the nematode-trapping fungus Arthrobotrys oligospora. Mycological Research, 96(3), 194–198. https://doi.org/10.1016/S0953-7562(09)80965-9
Decraemer, W., & Hunt, D. J. (2006). Structure and classification. In R. . Perry & M. Moens (Eds.), Plant nematology (pp. 3–32). CABI. https://doi.org/10.1079/9781845930561.0003
Degenkolb, T., & Vilcinskas, A. (2016). Metabolites from nematophagous fungi and nematicidal natural products from fungi as alternatives for biological control. Part II: metabolites from nematophagous basidiomycetes and non-nematophagous fungi. Applied Microbiology and Biotechnology, 100(9), 3813–3824. https://doi.org/10.1007/S00253-015-7234-5
Djian, C., Pijarouvski, L., Ponchet, M., & Arpin, N. (1991). Acetic acid, a selective nematicidal metabolite from culture filtrate of Paecilomyces lilacinus (Thom) Samsan and Trichoderma longibrachiatum Rifai. Nematologica, 37, 101–112.
Engelbrecht, G., Claassens, S., Mienie, C. M. S., & Fourie, H. (2022). Filtrates of mixed Bacillus spp inhibit second-stage juvenile motility of root-knot nematodes. Rhizosphere, 22, 100528. https://doi.org/10.1016/j.rhisph.2022.100528
Esikova, T. Z., Anokhina, T. O., Abashina, T. N., Suzina, N. E., & Solyanikova, I. P. (2021). Characterization of soil bacteria with potential to degrade benzoate and antagonistic to fungal and bacterial phytopathogens. Microorganisms, 9(4). https://doi.org/10.3390/MICROORGANISMS9040755
Fan, X.-J., Zhang, X., Zhang, F., Liu, S.-R., Su, X.-J., & Yang, X.-Y. (2018). Dactylellina parvicolla WZ27, a spontaneous conidial trap producing strain of nematode-trapping fungus. Mycosystema, 37(3), 305–313. https://doi.org/10.13346/J.MYCOSYSTEMA.170121
Fan, Y., Zhang, W., Chen, Y., Xiang, M., & Liu, X. (2021). DdaSTE12 is involved in trap formation, ring inflation, conidiation, and vegetative growth in the nematode-trapping fungus Drechslerella dactyloides. Applied Microbiology and Biotechnology, 105(19), 7379–7393. https://doi.org/10.1007/S00253-021-11455-Z/FIGURES/6
Fischer, R., & Requena, N. (2022). Small-secreted proteins as virulence factors in nematode-trapping fungi. Trends in Microbiology. https://doi.org/10.1016/J.TIM.2022.03.005
Ganeshan, S., Annaiyan, S., Somasundaram, P., Mannu, J., Kathithachalam, A., Shanmugam, H., & Arunachalam, A. (2024). Biomolecule repository of endophytic bacteria from guava serves as a key player in suppressing root- knot nematode, Meloidogyne enterolobii. Scientia Horticulturae, 324, 112627. https://doi.org/10.1016/J.SCIENTA.2023.112627
Gao, H., Qi, G., Yin, R., Zhang, H., Li, C., & Zhao, X. (2016). Bacillus cereus strain S2 shows high nematicidal activity against Meloidogyne incognita by producing sphingosine. Scientific Reports, 6. https://doi.org/10.1038/SREP28756
Geng, C., Nie, X., Tang, Z., Zhang, Y., Lin, J., Sun, M., & Peng, D. (2016). A novel serine protease, Sep1, from Bacillus firmus DS-1 has nematicidal activity and degrades multiple intestinal-associated nematode proteins. Scientific Reports, 6(1), 1–12. https://doi.org/10.1038/srep25012
Grunseich, J. M., Aguirre, N. M., Thompson, M. N., Ali, J. G., & Helms, A. M. (2021). Chemical cues from entomopathogenic nematodes vary across three species with different foraging strategies, triggering different behavioral responses in prey and competitors. Journal of Chemical Ecology, 47(10–11), 822. https://doi.org/10.1007/S10886-021-01304-8
Gu, Y. Q., Mo, M. H., Zhou, J. P., Zou, C. S., & Zhang, K. Q. (2007). Evaluation and identification of potential organic nematicidal volatiles from soil bacteria. Soil Biology and Biochemistry, 39(10), 2567–2575. https://doi.org/10.1016/J.SOILBIO.2007.05.011
Haarith, D., Bushley, K. E., & Chen, S. (2020). Fungal communities associated with Heterodera glycines and their potential in biological control: a current update. Journal of Nematology, 52(1), 2020–2042. https://doi.org/10.21307/JOFNEM-2020-022
Hahn, M. H., May De Mio, L. L., Kuhn, O. J., & Duarte, H. da S. S. (2019). Nematophagous mushrooms can be an alternative to control Meloidogyne javanica. Biological Control, 138, 104024. https://doi.org/10.1016/J.BIOCONTROL.2019.104024
Herrera-Cabrera, B. E., Delgado-Alvarado, A., Salgado-Garciglia, R., López-Valdez, L. G., Sánchez-Herrera, L. M., et al. (2024). Volatile organic compound produced by bacteria: characterization and application. Bacterial Secondary Metabolites, 177–196. https://doi.org/10.1016/B978-0-323-95251-4.00011-9
Hsueh, Y. P., Gronquist, M. R., Schwarz, E. M., Nath, R. D., Lee, C. H., Gharib, S., Schroeder, F. C., & Sternberg, P. W. (2017). Nematophagous fungus Arthrobotrys oligospora mimics olfactory cues of sex and food to lure its nematode prey. ELife, 6. https://doi.org/10.7554/ELIFE.20023
Hsueh, Y. P., Mahanti, P., Schroeder, F. C., & Sternberg, P. W. (2013). Nematode-trapping fungi eavesdrop on nematode pheromones. Current Biology : CB, 23(1), 83. https://doi.org/10.1016/J.CUB.2012.11.035
Huang, D., Yu, C., Shao, Z., Cai, M., Li, G., Zheng, L., Yu, Z., & Zhang, J. (2020). Identification and characterization of nematicidal volatile organic compounds from deep-sea Virgibacillus dokdonensis MCCC 1A00493. Molecules, 25(3). https://doi.org/10.3390/MOLECULES25030744
Ibrahim, S. R. M., Mohamed, S. G. A., Alsaadi, B. H., Althubyani, M. M., Awari, Z. I., et al. (2023). Secondary Metabolites, Biological Activities, and Industrial and Biotechnological Importance of Aspergillus sydowii. Marine Drugs, 21(8). https://doi.org/10.3390/MD21080441
Jiang, X., Xiang, M., & Liu, X. (2017). Nematode-trapping fungi. Microbiology Spectrum, 5(1). https://doi.org/10.1128/MICROBIOLSPEC.FUNK-0022-2016
Jiménez-Ortega, L. A., Orozco-Ochoa, A. K., Valdez-Baro, O., & Heredia, J. B. (2024). Microorganisms as biofactories of powerful agents against plant diseases. Entrepreneurship with Microorganisms, 1–15. https://doi.org/10.1016/B978-0-443-19049-0.00011-6
Ju, S., Lin, J., Zheng, J., Wang, S., Zhou, H., & Sun, M. (2016). Alcaligenes faecalis ZD02, a novel nematicidal bacterium with an extracellular serine protease virulence factor. Applied and Environmental Microbiology, 82(7), 2112–2120. https://doi.org/10.1128/AEM.03444-15
Kang, B. R., Anderson, A. J., & Kim, Y. C. (2018). Hydrogen cyanide produced by Pseudomonas chlororaphis O6 exhibits nematicidal activity against Meloidogyne hapla. The Plant Pathology Journal, 34(1), 35–43. https://doi.org/10.5423/PPJ.OA.06.2017.0115
Kassam, R., Yadav, J., Chawla, G., Kundu, A., Hada, A., Jaiswal, N., Bollinedi, H., Kamil, D., Devi, P., & Rao, U. (2021). Identification, characterization, and evaluation of nematophagous fungal species of Arthrobotrys and Tolypocladium for the management of Meloidogyne incognita. Frontiers in Microbiology, 12, 790223. https://doi.org/10.3389/FMICB.2021.790223
Kelliher, J. M., Robinson, A. J., Longley, R., Johnson, L. Y. D., Hanson, B. T., et al. (2023). The endohyphal microbiome: current progress and challenges for scaling down integrative multi-omic microbiome research. Microbiome, 11(1). https://doi.org/10.1186/S40168-023-01634-7
Khan, A., Haris, M., Hussain, T., Khan, A. A., Laasli, S. E., Lahlali, R., & Mokrini, F. (2023). Counter-attack of biocontrol agents: Environmentally benign Approaches against Root-knot nematodes (Meloidogyne spp.) on Agricultural crops. Heliyon, 9(11), e21653. https://doi.org/10.1016/J.HELIYON.2023.E21653
Khan, A., Williams, K. L., Soon, J., & Nevalainen, H. K. M. (2008). Proteomic analysis of the knob-producing nematode-trapping fungus Monacrosporium lysipagum. Mycological Research, 112(12), 1447–1452. https://doi.org/10.1016/J.MYCRES.2008.06.003
Khan, B., Yan, W., Wei, S., Wang, Z., Zhao, S., Cao, L., Rajput, N. A., & Ye, Y. (2019). Nematicidal metabolites from endophytic fungus Chaetomium globosum YSC5. FEMS Microbiology Letters, 366(14), fnz169. https://doi.org/10.1093/FEMSLE/FNZ169
Khan, R. A. A., Najeeb, S., Mao, Z., Ling, J., Yang, Y., Li, Y., & Xie, B. (2020). Bioactive secondary metabolites from Trichoderma spp. against phytopathogenic bacteria and root-knot nematode. Microorganisms, 8(3), 401. https://doi.org/10.3390/MICROORGANISMS8030401
Kirwa, H. K., Murungi, L. K., Beck, J. J., & Torto, B. (2018). Elicitation of differential responses in the root-knot nematode Meloidogyne incognita to tomato root exudate cytokinin, flavonoids, and alkaloids. Journal of Agricultural and Food Chemistry, 66(43), 11291–11300. https://doi.org/10.1021/ACS.JAFC.8B05101
Köhl, J., Kolnaar, R., & Ravensberg, W. J. (2019). Mode of action of microbial biological control agents against plant diseases: relevance beyond efficacy. Frontiers in Plant Science, 10. https://doi.org/10.3389/FPLS.2019.00845
Koziak, A. T. E., Cheng, K., & Thorn, R. G. (2007). Phylogenetic analyses of Nematoctonus and Hohenbuehelia (Pleurotaceae). Https://Doi.Org/10.1139/B07-083, 85(8), 762–773. https://doi.org/10.1139/B07-083
Kumar, K. K., & Dara, S. K. (2021). Fungal and bacterial endophytes as microbial control agents for plant-parasitic nematodes. International Journal of Environmental Research and Public Health, 18(8), 4269. https://doi.org/10.3390/IJERPH18084269
Kundu, A., & Vyshali, G. (2022). Current Status of Nematode-bacteria Interaction: A Mini Review. Agricultural Reviews. https://doi.org/10.18805/AG.R-2291
Kuo, T. H., Yang, C. T., Chang, H. Y., Hsueh, Y. P., & Hsu, C. C. (2020). Nematode-trapping fungi produce diverse metabolites during predator-prey interaction. Metabolites, 10(3). https://doi.org/10.3390/METABO10030117
Kusstatscher, P., Cernava, T., & Berg, G. (2020). Using bacteria-derived volatile organic compounds (VOCs) for industrial processes. Bacterial Volatile Compounds as Mediators of Airborne Interactions, 305–316. https://doi.org/10.1007/978-981-15-7293-7_13
Kwon, H. R., Son, S. W., Han, H. R., Choi, G. J., Jang, K. S., et al. (2007). Nematicidal activity of bikaverin and fusaric acid isolated from Fusarium oxysporum against pine wood nematode, Bursaphelenchus xylophilus. The Plant Pathology Journal, 23(4), 318–321. https://doi.org/10.5423/PPJ.2007.23.4.318
Lee, Y. S., & Kim, K. Y. (2016). Antagonistic potential of Bacillus pumilus L1 against root-knot nematode, Meloidogyne arenaria. Journal of Phytopathology, 164(1), 29–39. https://doi.org/10.1111/JPH.12421
Lee, Y. S., Nguyen, X. H., Naing, K. W., Park, Y. S., & Kim, K. Y. (2015). Role of lytic enzymes secreted by Lysobacter capsici YS1215 in the control of root-knot nematode of tomato plants. Indian Journal of Microbiology, 55(1), 74–80. https://doi.org/10.1007/S12088-014-0499-Z/TABLES/2
Leeder, A. C., Palma-Guerrero, J., & Glass, N. L. (2011). The social network: deciphering fungal language. Nature Reviews Microbiology 2011 9:6, 9(6), 440–451. https://doi.org/10.1038/nrmicro2580
Lei, H.-M., Wang, J.-T., Hu, Q.-Y., Li, C.-Q., Mo, M.-H., Zhang, K.-Q., Li, G.-H., & Zhao, P.-J. (2023). 2-Furoic acid associated with the infection of nematodes by Dactylellina haptotyla and its biocontrol potential on plant root-knot nematodes. Microbiology Spectrum, 11(5). https://doi.org/10.1128/SPECTRUM.01896-23/SUPPL_FILE/SPECTRUM.01896-23-S0003.XLSX
Li, G. H., & Zhang, K. Q. (2023). Natural nematicidal metabolites and advances in their biocontrol capacity on plant parasitic nematodes. Natural Product Reports, 40(3), 646–675. https://doi.org/10.1039/D2NP00074A
Li, G., Zhang, K., Xu, J., Dong, J., & Liu, Y. (2007). Nematicidal substances from fungi. Recent Patents on Biotechnology, 1(3), 212–233. https://doi.org/10.2174/187220807782330165
Li, J., Zou, C., Xu, J., Ji, X., Niu, X., Yang, J., Huang, X., & Zhang, K.-Q. (2015). Molecular mechanisms of nematode-nematophagous microbe interactions: basis for ciological Control of plant-parasitic nematodes. Annual Review of Phytopathology, 53, 67–95. https://doi.org/10.1146/ANNUREV-PHYTO-080614-120336
Li, L., Ma, M., Liu, Y., Zhou, J., Qu, Q., Lu, K., Fu, D., & Zhang, K. (2011). Induction of trap formation in nematode-trapping fungi by a bacterium. FEMS Microbiology Letters, 322(2), 157–165. https://doi.org/10.1111/J.1574-6968.2011.02351.X
Li, L., Yang, M., Luo, J., Qu, Q., Chen, Y., Liang, L., & Zhang, K. (2016). Nematode-trapping fungi and fungus-associated bacteria interactions: the role of bacterial diketopiperazines and biofilms on Arthrobotrys oligospora surface in hyphal morphogenesis. Environmental Microbiology, 18(11), 3827–3839. https://doi.org/10.1111/1462-2920.13340
Liang, L.-M., Zou, C.-G., Xu, J., & Zhang, K.-Q. (2019). Signal pathways involved in microbe–nematode interactions provide new insights into the biocontrol of plant-parasitic nematodes. Philosophical Transactions of the Royal Society B, 374(1767). https://doi.org/10.1098/RSTB.2018.0317
Liang, L., Liu, Z., Liu, L., Li, J., Gao, H., Yang, J., & Zhang, K. Q. (2016). The nitrate assimilation pathway is involved in the trap formation of Arthrobotrys oligospora, a nematode-trapping fungus. Fungal Genetics and Biology, 92, 33–39. https://doi.org/10.1016/J.FGB.2016.05.003
Liang, L. M., Zou, C. G., & Zhang, K. Q. (2019). Advances in the molecular mechanisms of the microbe-nematode interaction. SCIENTIA SINICA Vitae, 49(11), 1508–1519. https://doi.org/10.1360/SSV-2019-0144
Lipke, P. N. (2018). What we do not know about fungal cell adhesion molecules. Journal of Fungi 2018, Vol. 4, Page 59, 4(2), 59. https://doi.org/10.3390/JOF4020059
Liu, K., Zhang, W., Lai, Y., Xiang, M., Wang, X., Zhang, X., & Liu, X. (2014). Drechslerella stenobrocha genome illustrates the mechanism of constricting rings and the origin of nematode predation in fungi. BMC Genomics, 15, 114. https://doi.org/10.1186/1471-2164-15-114
Lopes, A. D., Rivadavea, W. R., & Silva, G. J. (2024). Trichoderma secondary metabolites for effective plant pathogen control. Nanohybrid Fungicides, 239–255. https://doi.org/10.1016/B978-0-443-23950-2.00008-4
Manan, A., Bazai, Z. A., Fan, J., Yu, H., & Li, L. (2018). The Nif3-family protein YqfO03 from Pseudomonas syringae MB03 has multiple nematicidal activities against Caenorhabditis elegans and Meloidogyne incognita. International Journal of Molecular Sciences, 19(12). https://doi.org/10.3390/IJMS19123915
Manohar, M., Tenjo-Castano, F., Chen, S., Zhang, Y. K., Kumari, A., Williamson, V. M., Wang, X., Klessig, D. F., & Schroeder, F. C. (2020). Plant metabolism of nematode pheromones mediates plant-nematode interactions. Nature Communications, 11(1), 1–11. https://doi.org/10.1038/s41467-019-14104-2
Martínez-Servat, S., Pinyol-Escala, L., Daura-Pich, O., Almazán, M., Hernández, I., López-García, B., & Fernández, C. (2023). Characterization of Lysobacter enzymogenes B25, a potential biological control agent of plant-parasitic nematodes, and its mode of action. AIMS Microbiology, 9(1), 151. https://doi.org/10.3934/MICROBIOL.2023010
Maulidia, V., Soesanto, L., Syamsuddin, Khairan, K., Hamaguchi, T., Hasegawa, K., & Sriwati, R. (2020). Secondary metabolites produced by endophytic bacteria against the root-knot nematode (Meloidogyne sp.). Biodiversitas Journal of Biological Diversity, 21(11), 5270–5275. https://doi.org/10.13057/BIODIV/D211130
Mei, X., Wang, X., & Li, G. (2021). Pathogenicity and volatile nematicidal metabolites from Duddingtonia flagrans against Meloidogyne incognita. Microorganisms, 9(11). https://doi.org/10.3390/MICROORGANISMS9112268/S1
Mendoza, A. R., Kiewnick, S., & Sikora, R. A. (2008). In vitro activity of Bacillus firmus against the burrowing nematode Radopholus similis, the root-knot nematode Meloidogyne incognita and the stem nematode Ditylenchus dipsaci. Biocontrol Science and Technology, 18(4), 377–389. https://doi.org/10.1080/09583150801952143
Migunova, V. D., & Sasanelli, N. (2021). Bacteria as biocontrol tool against phytoparasitic nematodes. Plants, 10(2), 1–15. https://doi.org/10.3390/PLANTS10020389
Naranjo-Morán, J., Vera-Morales, M., & Mora-González, A. (2021). Acumulaciones de hierro en agroecosistemas bananeros (Milagro, Ecuador): Una revisión bibliográfica de algunos factores que intervienen en la salud y nutrición del cultivo. Siembra, 8(2), e2680. https://doi.org/10.29166/SIEMBRA.V8I2.2680
Niu, Q., Huang, X., Zhang, L., Xu, J., Yang, D., Wei, K., Niu, X., An, Z., Bennett, J. W., Zou, C., Yang, J., & Zhang, K. Q. (2010). A Trojan horse mechanism of bacterial pathogenesis against nematodes. Proceedings of the National Academy of Sciences of the United States of America, 107(38), 16631–16636. https://doi.org/10.1073/PNAS.1007276107/-/DCSUPPLEMENTAL
Nwokolo, N. L., Enebe, M. C., Chigor, C. B., Chigor, V. N., & Dada, O. A. (2021). The contributions of biotic lines of defence to improving plant disease suppression in soils: A review. Rhizosphere, 19, 100372. https://doi.org/10.1016/J.RHISPH.2021.100372
Ocampo-Gutiérrez, A. Y., Hernández-Velázquez, V. M., Aguilar-Marcelino, L., Cardoso-Taketa, A., Zamilpa, A., López-Arellano, M. E., González-Cortázar, M., Hernández-Romano, J., Reyes-Estebanez, M., & Mendoza-de Gives, P. (2021). Morphological and molecular characterization, predatory behaviour and effect of organic extracts of four nematophagous fungi from Mexico. Fungal Ecology, 49, 101004. https://doi.org/10.1016/J.FUNECO.2020.101004
Okorie, C. C., Ononuju, C. C., & Okwujiako, I. A. (2011). Management of Meloidogyne incognita with Pleurotus ostreatus and P. tuberregium in Soybean. International Journal of Agriculture & Biology, 401–405.
Park, Y. S., Dutta, S., Ann, M., Raaijmakers, J. M., & Park, K. (2015). Promotion of plant growth by Pseudomonas fluorescens strain SS101 via novel volatile organic compounds. Biochemical and Biophysical Research Communications, 461(2), 361–365. https://doi.org/10.1016/J.BBRC.2015.04.039
Poveda, J., Abril-Urias, P., & Escobar, C. (2020). Biological control of plant-parasitic nematodes by filamentous fungi inducers of resistance: Trichoderma, mycorrhizal and endophytic fungi. Frontiers in Microbiology, 11, 992. https://doi.org/10.3389/FMICB.2020.00992/BIBTEX
Quevedo, A., Magdama, F., Castro, J., & Vera-Morales, M. (2022). Interacciones ecológicas de los hongos nematófagos y su potencial uso en cultivos tropicales. Scientia Agropecuaria, 13(1), 97–108. https://doi.org/10.17268/SCI.AGROPECU.2022.009
Quevedo, A., Vera-Morales, M., Espinoza-Lozano, F., Castañeda-Ruiz, R. F., Sosa del Castillo, D., & Magdama, F. (2021). Assessing the predatory activity of Arthrobotrys oligosporus strain C-2197 as biocontrol of the root-knot nematode Meloidogyne spp. Bionatura, 6(1), 1586–1592. https://doi.org/10.21931/RB/2021.06.01.22
Raza, W., Wang, J., Jousset, A., Friman, V. P., Mei, X., Wang, S., Wei, Z., & Shen, Q. (2020). Bacterial community richness shifts the balance between volatile organic compound-mediated microbe–pathogen and microbe–plant interactions. Proceedings of the Royal Society B: Biological Sciences, 287(1925). https://doi.org/10.1098/RSPB.2020.0403
Rucker, C. J., & Zachariah, K. (1986). The influence of bacteria on trap induction in predacious hyphomycetes. Canadian Journal of Botany, 65(6), 1160–1162. https://doi.org/10.1139/B87-162
Sahebani, N., & Gholamrezaee, N. (2021). The biocontrol potential of Pseudomonas fluorescens CHA0 against root knot nematode (Meloidogyne javanica) is dependent on the plant species. Biological Control, 152, 104445. https://doi.org/10.1016/J.BIOCONTROL.2020.104445
Sánchez, V. H., & Zambrano, J. (2019). Adoption and impact of agricultural technologies developed in Ecuador. La Granja: Revista de Ciencias de La Vida , 30(2), 28–39. https://doi.org/10.17163/LGR.N30.2019.03
Saritha, M., Kumar, P., Panwar, N. R., & Burman, U. (2021). Intelligent plant–microbe interactions. Archives of Agronomy and Soil Science. https://doi.org/10.1080/03650340.2020.1870677
Sheoran, N., Valiya Nadakkakath, A., Munjal, V., Kundu, A., Subaharan, K., et al. (2015). Genetic analysis of plant endophytic Pseudomonas putida BP25 and chemo-profiling of its antimicrobial volatile organic compounds. Microbiological Research, 173, 66–78. https://doi.org/10.1016/J.MICRES.2015.02.001
Siddiqui, Z. A., & Aziz, S. (2024). Plant parasitic nematode-fungus interactions: recent concepts and mechanisms. Plant Physiology Reports, 1, 1–14. https://doi.org/10.1007/S40502-023-00762-4/TABLES/2
Singh, P., & Siddiqui, Z. A. (2009). Biocontrol of root-knot nematode Meloidogyne incognita by the isolates of Bacillus on tomato. Archives of Phytopathology and Plant Protection, 43(6), 552–561. https://doi.org/10.1080/03235400801939904
Soliman, G. M., Ameen, H. H., Abdel-Aziz, S. M., & El-Sayed, G. M. (2019). In vitro evaluation of some isolated bacteria against the plant parasite nematode Meloidogyne incognita. Bulletin of the National Research Centre, 43(1), 1–7. https://doi.org/10.1186/S42269-019-0200-0
Soliman, M. S., El-Deriny, M. M., Ibrahim, D. S. S., Zakaria, H., & Ahmed, Y. (2021). Suppression of root-knot nematode Meloidogyne incognita on tomato plants using the nematode trapping fungus Arthrobotrys oligospora Fresenius. Journal of Applied Microbiology. https://doi.org/10.1111/jam.15101
Su, H. N., Xu, Y. Y., Wang, X., Zhang, K. Q., & Li, G. H. (2016). Induction of trap formation in nematode-trapping fungi by bacteria-released ammonia. Letters in Applied Microbiology, 62(4), 349–353. https://doi.org/10.1111/lam.12557
Su, H., Zhao, Y., Zhou, J., Feng, H., Jiang, D., Zhang, K.-Q., & Yang, J. (2017). Trapping devices of nematode-trapping fungi: formation, evolution, and genomic perspectives. Biological Reviews of the Cambridge Philosophical Society, 92(1), 357–368. https://doi.org/10.1111/brv.12233
Sujayanand, G. K., Chandra, A., Jagadeeswaran, R., Rout, A. K., Kumar, S., & Dubey, S. (2024). Bacillus and Related Genera on Biocontrol of Insects and Nematodes. In V. Mageshwaran, U. B. Singh, A. K. Saxena, & H. . Singh (Eds.), Applications of Bacillus and Bacillus Derived Genera in Agriculture, Biotechnology and Beyond. Microorganisms for Sustainability (pp. 151–164). Springer, Singapore. https://doi.org/10.1007/978-981-99-8195-3_8
Sun, Y., Ran, Y., Yang, H., Mo, M., & Li, G. (2023). Volatile Metabolites from Brevundimonas diminuta and Nematicidal Esters Inhibit Meloidogyne javanica. Microorganisms, 11(4), 966. https://doi.org/10.3390/MICROORGANISMS11040966/S1
Thakur, S., & Zachariah, K. (1989). Response of the fungus Dactylella rhopalota to bacteria. Plant and Soil, 120(1), 87–93. https://doi.org/10.1007/BF02370294
Thorn, R. G., Moncalvo, J.-M., Reddy, C. A., & Vilgalys, R. (2000). Phylogenetic analyses and the distribution of nematophagy support a monophyletic Pleurotaceae within the polyphyletic pleurotoid-lentinoid fungi. Mycologia, 92(2), 241–252. https://doi.org/10.1080/00275514.2000.12061151
Tong, S. M., & Feng, M. G. (2019). Insights into regulatory roles of MAPK-cascaded pathways in multiple stress responses and life cycles of insect and nematode mycopathogens. Applied Microbiology and Biotechnology, 103(2), 577–587. https://doi.org/10.1007/S00253-018-9516-1/TABLES/2
Vera-Morales, M., Castañeda-Ruiz, R. F., Sosa, D., Quevedo, A., Naranjo-Morán, J., Serrano, L., & Ratti, M. F. (2022). Mecanismos de captura, colonización y alimentación empleados por parásitos y predadores de nematodos: Ecosistemas, 31(3), 2390. https://doi.org/10.7818/ECOS.2390
Vera-Morales, M., López Medina, S. E., Naranjo-Morán, J., Quevedo, A., & Ratti, M. F. (2023). Nematophagous Fungi: A Review of Their Phosphorus Solubilization Potential. Microorganisms, 11(1), 137. https://doi.org/10.3390/microorganisms11010137
Wang, B.-L., Chen, Y.-H., He, J.-N., Xue, H.-X., Yan, N., et al. (2018). Integrated metabolomics and morphogenesis reveal volatile signaling of the nematode-trapping fungus Arthrobotrys oligospora. Applied and Environmental Microbiology, 84(9), e02749-17. https://doi.org/10.1128/AEM.02749-17
Wang, X., Li, G. H., Zou, C. G., Ji, X. L., Liu, T., et al. (2014). Bacteria can mobilize nematode-trapping fungi to kill nematodes. Nature Communications 2014 5:1, 5(1), 1–9. https://doi.org/10.1038/ncomms6776
Westerhoff, H. V., Brooks, A. N., Simeonidis, E., García-Contreras, R., He, F., et al. (2014). Macromolecular networks and intelligence in microorganisms. Frontiers in Microbiology, 5(7), 379. https://doi.org/10.3389/FMICB.2014.00379/BIBTEX
Wheatley, R. E. (2002). The consequences of volatile organic compound mediated bacterial and fungal interactions. Antonie van Leeuwenhoek 2002 81:1, 81(1), 357–364. https://doi.org/10.1023/A:1020592802234
Witzany, G. (2017). Key levels of biocommunication. In Biocommunication: sign-mediated interactions between cells and organisms (pp. 37–62). World Scientific Publishing Co. Pte. Ltd. https://doi.org/10.1142/9781786340450_0002
Wolfgang, A., Taffner, J., Guimarães, R. A., Coyne, D., & Berg, G. (2019). Novel strategies for soil-borne diseases: Exploiting the microbiome and volatile-based mechanisms toward controlling Meloidogyne-based disease complexes. Frontiers in Microbiology, 10. https://doi.org/10.3389/FMICB.2019.01296/FULL
Xiong, J., Zhou, Q., Luo, H., Xia, L., Li, L., Sun, M., & Yu, Z. (2015). Systemic nematicidal activity and biocontrol efficacy of Bacillus firmus against the root-knot nematode Meloidogyne incognita. World Journal of Microbiology and Biotechnology, 31(4), 661–667. https://doi.org/10.1007/S11274-015-1820-7/TABLES/1
Xu, L. L., Lai, Y. L., Wang, L., & Liu, X. Z. (2011). Effects of abscisic acid and nitric oxide on trap formation and trapping of nematodes by the fungus Drechslerella stenobrocha AS6.1. Fungal Biology, 115(2), 97–101. https://doi.org/10.1016/J.FUNBIO.2010.10.006
Xu, Y. Y., Lu, H., Wang, X., Zhang, K. Q., & Li, G. H. (2015). Effect of volatile organic compounds from bacteria on nematodes. Chemistry & Biodiversity, 12(9), 1415–1421. https://doi.org/10.1002/CBDV.201400342
Yang, C. T., de Ulzurrun, G. V. D., Pedro Gonçalves, A., Lin, H. C., Chang, C. W., et al. (2020). Natural diversity in the predatory behavior facilitates the establishment of a robust model strain for nematode-trapping fungi. Proceedings of the National Academy of Sciences of the United States of America, 117(12), 6762–6770. https://doi.org/10.1073/PNAS.1919726117/-/DCSUPPLEMENTAL
Yang, X., Yu, H., Ren, J., Cai, L., Xu, L., & Liu, L. (2023). Sulfoxide-Containing Bisabolane Sesquiterpenoids with Antimicrobial and Nematicidal Activities from the Marine-Derived Fungus Aspergillus sydowii LW09. Journal of Fungi, 9(3). https://doi.org/10.3390/JOF9030347/S1
Yin, N., Liu, R., Zhao, J. L., Khan, R. A. A., Li, Y., et al. (2021). Volatile organic compounds of Bacillus cereus strain Bc-cm103 exhibit fumigation activity against Meloidogyne incognita. Plant Disease, 105(4), 904–911. https://doi.org/10.1094/PDIS-04-20-0783-RE
Youssar, L., Wernet, V., Hensel, N., Yu, X., Hildebrand, H. G., et al. (2019). Intercellular communication is required for trap formation in the nematode-trapping fungus Duddingtonia flagrans. PLOS Genetics, 15(3), e1008029. https://doi.org/10.1371/JOURNAL.PGEN.1008029
Yu, J. M., Wang, D., Pierson, L. S., & Pierson, E. A. (2018). Effect of producing different phenazines on bacterial fitness and biological control in Pseudomonas chlororaphis 30-84. The Plant Pathology Journal, 34(1), 44. https://doi.org/10.5423/PPJ.FT.12.2017.0277
Yu, X., Hu, X., Pop, M., Wernet, N., Kirschhöfer, F., Brenner-Weiß, G., Keller, J., Bunzel, M., & Fischer, R. (2021). Fatal attraction of Caenorhabditis elegans to predatory fungi through 6-methyl-salicylic acid. Nature Communications 2021 12:1, 12(1), 1–10. https://doi.org/10.1038/s41467-021-25535-1
Zhai, Y., Shao, Z., Cai, M., Zheng, L., Li, G., et al. (2018). Multiple modes of nematode control by volatiles of Pseudomonas putida 1A00316 from antarctic soil against Meloidogyne incognita. Frontiers in Microbiology, 9(FEB). https://doi.org/10.3389/FMICB.2018.00253
Zhai, Y., Shao, Z., Cai, M., Zheng, L., Li, G., Yu, Z., & Zhang, J. (2019). Cyclo(l-Pro–l-Leu) of Pseudomonas putida MCCC 1A00316 isolated from antarctic soil: identification and characterization of activity against Meloidogyne incognita. Molecules, 24(4). https://doi.org/10.3390/MOLECULES24040768
Zhang, D., Zhu, X., Sun, F., Zhang, K., Niu, S., & Huang, X. (2017). The roles of actin cytoskeleton and actin-associated protein Crn1p in trap formation of Arthrobotrys oligospora. Research in Microbiology, 168(7), 655–663. https://doi.org/10.1016/J.RESMIC.2017.05.001
Zhang, F., Peng, D., Ye, X., Yu, Z., Hu, Z., Ruan, L., & Sun, M. (2012). In vitro uptake of 140 kDa Bacillus thuringiensis nematicidal crystal proteins by the second stage juvenile of Meloidogyne hapla. PLOS ONE, 7(6), e38534. https://doi.org/10.1371/journal.pone.0038534
Zhang, X., Zhang, H., Jiang, Z., Bai, Q., Wu, S., et al. (2021). A new strain of Volutella citrinella with nematode predation and nematicidal activity, isolated from the cysts of potato cyst nematodes in China. BMC Microbiology, 21(1). https://doi.org/10.1186/S12866-021-02385-X
Zhao, X., Lin, C., Tan, J., Yang, P., Wang, R., & Qi, G. (2023). Changes of rhizosphere microbiome and metabolites in Meloidogyne incognita infested soil. Plant and Soil, 483(1–2), 331–353. https://doi.org/10.1007/S11104-022-05742-5/FIGURES/6
Zheng, H., Chen, T., Li, W., Hong, J., Xu, J., & Yu, Z. (2024). Endosymbiotic bacteria within the nematode-trapping fungus Arthrobotrys musiformis and their potential roles in nitrogen cycling. Frontiers in Microbiology, 15. https://doi.org/10.3389/FMICB.2024.1349447/FULL
Zheng, L., Li, G., Wang, X., Pan, W., Li, L., et al. (2008). Nematicidal endophytic bacteria obtained from plants. Annals of Microbiology 2008 58:4, 58(4), 569–572. https://doi.org/10.1007/BF03175559
Publicado
Cómo citar
Número
Sección
Licencia
Derechos de autor 2024 Scientia Agropecuaria

Esta obra está bajo una licencia internacional Creative Commons Atribución-NoComercial 4.0.
Los autores que publican en esta revista aceptan los siguientes términos:
a. Los autores conservan los derechos de autor y conceden a la revista el derecho publicación, simultáneamente licenciada bajo una licencia de Creative Commons que permite a otros compartir el trabajo, pero citando la publicación inicial en esta revista.
b. Los autores pueden celebrar acuerdos contractuales adicionales separados para la distribución no exclusiva de la versión publicada de la obra de la revista (por ejemplo, publicarla en un repositorio institucional o publicarla en un libro), pero citando la publicación inicial en esta revista.
c. Se permite y anima a los autores a publicar su trabajo en línea (por ejemplo, en repositorios institucionales o en su sitio web) antes y durante el proceso de presentación, ya que puede conducir a intercambios productivos, así como una mayor citación del trabajo publicado (ver efecto del acceso abierto).




