Incidence of Moniliophthora roreri pathogen of cocoa in Ecuador and chemical management at in vitro and in vivo levels
DOI:
https://doi.org/10.17268/sci.agropecu.2026.018Keywords:
isolation, inhibition, moniliasis, sensitivity, severityAbstract
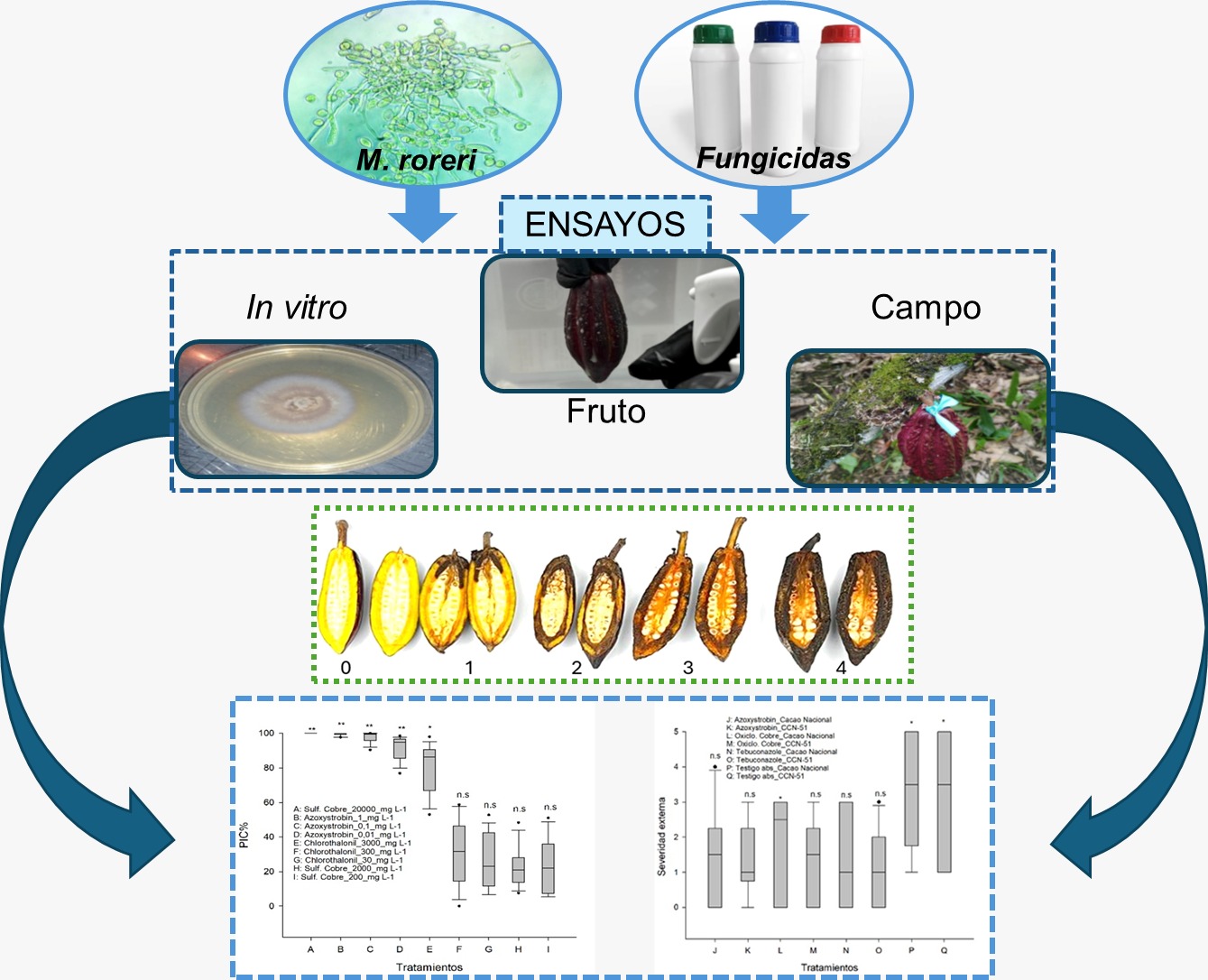
The phytopathogen M. roreri represents one of the main phytosanitary problems worldwide, it is still a challenge and represents a risk for almond production. The efficient use of fungicides reduces the impact on the environment and contributes to the care for the non-resistance of the pathogen. This work carried out an experimental study to estimate the capacity of chemical fungicides for in vitro and in vivo management of the disease and also analyzed the effective isolates of the pathogen that have been reported in laboratory conditions in Ecuador. Three experimental trials were carried out, the first with a DCA to estimate effective doses of azoxystrobin, copper sulfate pentahydrate and chlorothalonil under in vitro conditions; the second a factorial DCA to evaluate the effect of antifungals on loose fruits inoculated with the pathogen under controlled conditions using conidia spraying; and a DCL to study the effect of combined fungicides for pathogen decline under field conditions, the variables evaluated were: growth area, PIC in Petri boxes, incidence, external severity on cocoa fruits. Copper sulfate and azoxystrobin showed high efficacy in vitro against M. roreri, with control greater than 90%. In the field, fungicide combinations significantly reduced the incidence and severity of the pathogen in cocoa; loose cocoa pods were not suitable for evaluating virulence or antifungal efficacy. In the incidence analysis, 59.09% of M. roreri isolates at the in vitro level did not show significant genetic differences in Ecuador.
References
Acquaroni, M., Svartz, G., & Pérez Coll, C. (2021). Developmental Toxicity Assessment of a Chlorothalonil-Based Fungicide in a Native Amphibian Species. Archives of Environmental Contamination and Toxicology, 80(4), 680-690. https://doi.org/10.1007/s00244-020-00734-x
Aguirre Cobos, G. X. (2019). Caracterización molecular de Moniliophthora roreri causante de la vaina helada (moniliasis) en el cacao en tres provincias del Ecuador: Los Ríos, Manabí y Santo Domingo de los Tsáchilas [master Thesis, Quito]. http://repositorio.usfq.edu.ec/handle/23000/7780
Ali, S. S., Shao, J., Strem, M. D., Phillips-Mora, W., Zhang, D., Meinhardt, L. W., & Bailey, B. A. (2015). Combination of RNAseq and SNP nanofluidic array reveals the center of genetic diversity of cacao pathogen Moniliophthora roreri in the upper Magdalena Valley of Colombia and its clonality. Frontiers in Microbiology, 6, 850. https://doi.org/10.3389/fmicb.2015.00850
Al-Shuhaib, M. B. S., & Hashim, H. O. (2023). Mastering DNA chromatogram analysis in Sanger sequencing for reliable clinical analysis. Journal of Genetic Engineering and Biotechnology, 21(1), 115. https://doi.org/10.1186/s43141-023-00587-6
Alvarado, J., Restrepo-Arias, J. F., Velásquez, D., Maiza, M., Alvarado, J., Restrepo-Arias, J. F., Velásquez, D., & Maiza, M. (2025). Disease Detection on Cocoa Crops Based on Computer-Vision Techniques: A Systematic Literature Review. Agriculture, 15(10). https://doi.org/10.3390/agriculture15101032
Alves da Silva, N. J., Menezes Reis, S. P., Diorato, V. S., Rocha, J. S. A., Barbosa, C. S., Ciampi-Guillardi, M., Patrocínio, N. G. R. B., Niella, G. R., Solis, K., Peñaherrera, S., Manco, M. J. da S., Teixeira, G. A., Arévalo-Gardini, E., & Gramacho, K. P. (2022). A molecular diagnostic for Moniliophthora perniciosa, the causal agent of witches’ broom disease of cacao, that differentiates it from its sister taxon Moniliophthora roreri. Crop Protection, 158, 106003. https://doi.org/10.1016/j.cropro.2022.106003
Amaya Márquez, D. J., & Pérez Martínez, S. (2020). Análisis de la estructura poblacional de Monillophthora roreri mediante marcadores genéticos SSR y fenotípicos de sensibilidad a fungicidas y agresividad en Theobroma cacao L [Thesis, ESPOL. FCV.]. http://www.dspace.espol.edu.ec/handle/123456789/55249
Amaya-Márquez, D. J., Espinoza-Lozano, F., Villavicencio-Vásquez, M. E., Castillo, D. S. del, & Pérez-Martínez, S. (2022). Inhibición y estimulación del crecimiento micelial de Moniliophthora roreri por flutolanil en poblaciones de Ecuador. Acta Agronómica, 70(3), Article 3. https://doi.org/10.15446/acag.v70n3.88905
Bailey, B. A., Evans, H. C., Phillips-Mora, W., Ali, S. S., & Meinhardt, L. W. (2018). Moniliophthora roreri, causal agent of cacao frosty pod rot. Molecular Plant Pathology, 19(7), 1580-1594. https://doi.org/10.1111/mpp.12648
Bamisile, B. S., Dash, C. K., Akutse, K. S., Keppanan, R., Afolabi, O. G., Hussain, M., Qasim, M., & Wang, L. (2018). Prospects of endophytic fungal entomopathogens as biocontrol and plant growth promoting agents: An insight on how artificial inoculation methods affect endophytic colonization of host plants. Microbiological Research, 217, 34-50. https://doi.org/10.1016/j.micres.2018.08.016
Bateman, R. p., Hidalgo, E., García, J., Arroyo, C., Ten Hoopen, G. m., Adonijah, V., & Krauss, U. (2005). Application of chemical and biological agents for the management of frosty pod rot (Moniliophthora roreri) in Costa Rica cocoa (Theobroma cacao). Annals of Applied Biology, 147(2), 129-138. https://doi.org/10.1111/j.1744-7348.2005.00012.x
Bryceson, S. R., Morgan, J. W., McMahon, P. J., & Keane, P. J. (2023). A sudden and widespread change in symptoms and incidence of vascular streak dieback of cocoa (Theobroma cacao) linked to environmental change in Sulawesi, Indonesia. Agriculture, Ecosystems & Environment, 350, 108466. https://doi.org/10.1016/j.agee.2023.108466
Ceccarelli, V., Fremout, T., Chavez, E., Argüello, D., Loor Solórzano, R. G., Sotomayor Cantos, I. A., & Thomas, E. (2024). Vulnerability to climate change of cultivated and wild cacao in Ecuador. https://doi.org/10.1007/s10584-024-03756-9
Correa Álvarez, J., Castro Martínez, S., & Coy, J. (2014). Estado de la moniliasis del cacao causada por Moniliophthora roreri en Colombia. Acta Agronómica, 63(4), 388-399. https://doi.org/10.15446/acag.v63n4.42747
da Costa, G. A., Lira, J. B., Freitas-Lopes, R. do L., & Lopes, U. P. (2019). Tank mix application of copper hydroxide either with cyproconazole or pyraclostrobin fungicides reduced the control of coffee leaf rust. Crop Protection, 124, 104856. https://doi.org/10.1016/j.cropro.2019.104856
Delgado-Bogotá, N. V., Patiño-Ladino, O. J., Prieto-Rodríguez, J. A., Delgado-Bogotá, N. V., Patiño-Ladino, O. J., & Prieto-Rodríguez, J. A. (2025). Antifungal Potential of Piper-Derived Essential Oils and Key Constituents on Moniliophthora roreri, the Causal Agent of Moniliasis in Cacao (Theobroma cacao L.). Plants, 14(16). https://doi.org/10.3390/plants14162514
Di Rienzo, J. A., Casanoves, F., Balzarini, M. G., Gonzalez, L., Tablada, M., & Robledo, C. W. (2020). InfoStat versión 2020. Centro de Transferencia InfoStat, FCA, Universidad Nacional de Córdoba, Argentina. URL http://www. infostat. com. ar.
Edgington, L. V. (1971). Fungitoxic Spectrum of Benzimidazole Compounds. Phytopathology, 61(1), 42. https://doi.org/10.1094/Phyto-61-42
Espinoza-Lozano, F., Amaya-Márquez, D., Pinto, C. M., Villavicencio-Vásquez, M., Sosa del Castillo, D., & Pérez-Martínez, S. (2022). Multiple Introductions of Moniliophthora roreri from the Amazon to the Pacific Region in Ecuador and Shared High Azoxystrobin Sensitivity. Agronomy, 12(5), Article 5. https://doi.org/10.3390/agronomy12051119
Evans, H. C., Stalpers, J. A., Samson, R. A., & Benny, G. L. (1978). On the taxonomy of Monilia roreri, an important pathogen of Theobroma cacao in South America. Canadian Journal of Botany, 56(20), 2528-2532. https://doi.org/10.1139/b78-305
Ezziyyani, M., Sánchez, C. P., Requena, M. E., Rubio, L., & Castillo, M. E. C. (2004). Biocontrol por Streptomyces roche – ziyani–, de la podredumbre del pimiento (Capsicum annuum L.) causada por Phytophthora capsici. Anales de Biología, 26, Article 26.
Gómez-Vázquez, E. G., Roque, Y. S., Ibáñez-Duharte, G. R., Canseco-Pérez, M. A., Zenteno-Carballo, A. G., Berrones-Hernández, R., & Pérez-Luna, Y. C. (2024). Molecular identification and lipolytic potential of filamentous fungi isolated from residual cooking oil. Biodiversity Data Journal, 12, e113698. https://doi.org/10.3897/BDJ.12.e113698
Guevara-Viejó, F., Valenzuela-Cobos, J. D., Noriega-Verdugo, D., Garcés-Moncayo, M. F., Quilligana, R. B., Guevara-Viejó, F., Valenzuela-Cobos, J. D., Noriega-Verdugo, D., Garcés-Moncayo, M. F., & Quilligana, R. B. (2024). Application of biplot techniques to evaluate the potential of Trichoderma spp. as a biological control of moniliasis in Ecuadorian cacao. Applied Sciences, 14(13). https://doi.org/10.3390/app14135481
Jiang, C., Zhou, L., Wang, M., Shen, S., Cheng, W., Zhao, Q., Cui, K., & He, L. (2025). Sensitivity determination and resistance mechanism of Sclerotium rolfsii to difenoconazole. Pest Management Science, 81(6), 2734-2741. https://doi.org/10.1002/ps.8624
Laflamme, B., Blackman, C., Loranger, M., Trilles, R., Doytchinova-Weil, K., Scully, S. S., Blackburn, J. M., Kanegesuku, A. L. G., Roizen, J. L., Bengtson, S., Brown, L. E., Porco, J. A., Yoshioka, K., Whitesell, L., Subramaniam, R., Robbins, N., & Cowen, L. E. (2025a). A cationic amphiphilic drug synergizes with strobilurin fungicides to control fungal-borne plant diseases. Cell Chemical Biology, 32(6), 872-884.e7. https://doi.org/10.1016/j.chembiol.2025.05.008
Laflamme, B., Blackman, C., Loranger, M., Trilles, R., Doytchinova-Weil, K., Scully, S. S., Blackburn, J. M., Kanegesuku, A. L. G., Roizen, J. L., Bengtson, S., Brown, L. E., Porco, J. A., Yoshioka, K., Whitesell, L., Subramaniam, R., Robbins, N., & Cowen, L. E. (2025b). A cationic amphiphilic drug synergizes with strobilurin fungicides to control fungal-borne plant diseases. Cell Chemical Biology, 32(6), 872-884.e7. https://doi.org/10.1016/j.chembiol.2025.05.008
Love, J., Selker, R., Marsman, M., Jamil, T., Dropmann, D., Verhagen, J., Ly, A., Gronau, Q. F., Šmíra, M., Epskamp, S., Matzke, D., Wild, A., Knight, P., Rouder, J. N., Morey, R. D., & Wagenmakers, E.-J. (2019). JASP: Graphical Statistical Software for Common Statistical Designs. Journal of Statistical Software, 88, 1-17. https://doi.org/10.18637/jss.v088.i02
Maridueña-Zavala, M. G., Villavicencio-Vásquez, M. E., Cevallos-Cevallos, J. M., & Peralta, E. L. (2016). Molecular and morphological characterization of Moniliophthora roreri isolates from cacao in Ecuador. Canadian Journal of Plant Pathology, 38(4), 460-469. https://doi.org/10.1080/07060661.2016.1261372
Márquez, D. A., Vásquez, A. L., Thompson, J. M., Párraga, D. A., Murillo, A. Á., Romero, K. C., & Asang, S. F. (2024). Efectividad fitosanitaria de la remoción de frutos enfermos con embolse para la disminución de Moniliophthora roreri en cultivo de cacao (Theobroma cacao L.). Pro Sciences: Revista de Producción, Ciencias e Investigación, 8(54), 1-11. https://doi.org/10.29018/issn.2588-1000vol8iss54.2024pp1-11
Mendoza, D., García, O., Velasco, J. L. R., & Jadán, K. (2024). Exportaciones de cacao ecuatoriano y su incidencia en la producción durante el período 2008-2023. Religación, 9(42), e2401278-e2401278. https://doi.org/10.46652/rgn.v9i42.1278
Ons, L., Bylemans, D., Thevissen, K., & Cammue, B. P. A. (2020). Combining Biocontrol Agents with Chemical Fungicides for Integrated Plant Fungal Disease Control. Microorganisms, 8(12), Article 12. https://doi.org/10.3390/microorganisms8121930
Özdemir, Ö. E., Bretzel, T., Gfüllner, L., Gorjian, S., Katircioglu, Y., Dur, B., & Trommsdorff, M. (2025). Design, simulation, and experimental evaluation of an agrivoltaic greenhouse in Turkey. Results in Engineering, 26, 105278. https://doi.org/10.1016/j.rineng.2025.105278
Phillips-Mora, W., Aime, M. C., & Wilkinson, M. J. (2007). Biodiversity and biogeography of the cacao (Theobroma cacao) pathogen Moniliophthora roreri in tropical America. Plant Pathology, 56(6), 911-922. https://doi.org/10.1111/j.1365-3059.2007.01646.x
Phillips-Mora, W., Castillo, J., Krauss, U., Rodríguez, E., & Wilkinson, M. J. (2005). Evaluation of cacao (Theobroma cacao) clones against seven Colombian isolates of Moniliophthora roreri from four pathogen genetic groups. Plant Pathology, 54(4), 483-490. https://doi.org/10.1111/j.1365-3059.2005.01210.x
Quiroga Pérez, M. (2022). Intraspecific variability of the Moniliophthora roreri populations in Colombia and its association with colony morphology and growth rate. http://hdl.handle.net/10784/31618
Rorer, J. B., & Pachano, A. (1918). Enfermedades del cacao en el Ecuador y métodos modernos apropiados a su cultivo: Informe presentado al Presidente y miembros de la Asociación de Agricultores del Ecuador. Imprenta de Diario llustrado.
Rudgard, S. A., Pettitt, T. R., & Hadley, P. (1990). Tenacity, biological activity and redistribution of copper fungicides on cocoa in controlled environments. Crop Protection, 9(4), 281-288. https://doi.org/10.1016/0261-2194(90)90106-H
Sánchez-Hernández, E., de la Cruz-Lázaro, E., & Sánchez-Hernández, R. (2014). Comportamiento de la moniliasis del cacao causada por Moniliophthora roreri (Cif. y Par.) en Tapachula, Chiapas, México. Acta Agrícola y Pecuaria, 1(1), 7-15.
Suárez Contreras, L. Y. (2016). Identificación molecular de aislamientos de Moniliophthora roreri en huertos de cacao de Norte de Santander, Colombia. Acta Agronómica, 65(1), 51-57. https://doi.org/10.15446/acag.v65n1.47994
Suárez Contreras, L. Y., & Rangel Riaño, A. L. (2013). Aislamiento de microorganismos para control biológico de Moniliophthora roreri. Acta Agronómica, 62(4), 370-378.
Torres de la Cruz, M., Ortiz García, C. F., Téliz Ortiz, D., Mora Aguilera, A., & Nava Díaz, C. (2013). Efecto del Azoxystrobin Sobre Moniliophthora roreri, Agente Causal de la Moniliasis del Cacao (Theobroma cacao). Revista mexicana de fitopatología, 31(1), 65-69.
Torres-de-la-Cruz, M., Quevedo-Damián, I., Ortiz-García, C. F., Lagúnez-Espinoza, L. D. C., Nieto-Angel, D., & Pérez-de La Cruz, M. (2019). Control químico de Moniliophthora roreri en México. Biotecnia, 21(2), 55-61. https://doi.org/10.18633/biotecnia.v21i2.906
Vela-Alvarez, E. L., Pérez-Gonzales, T. M., & Sánche, L. A. O. (2025). Manejo preventivo de moniliasis, mediante el uso de agua y aceite ozonizados. Revista Amazónica de Ciencias Ambientales y Ecológicas, 4(2), e859-e859. https://doi.org/10.51252/reacae.v4i2.859
Vélez Balderramo, E. T., Almeida Vera, D. A., & Vélez Zambrano, S. M. (2023). Efecto de fungicidas sistémicos y protectores en el control de moniliasis y escoba de bruja en cacao. https://agris.fao.org/search/en/providers/124692/records/669e7a4c00eb85b7d72b8a68
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Scientia Agropecuaria

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
The authors who publish in this journal accept the following conditions:
a. The authors retain the copyright and assign to the magazine the right of the first publication, with the work registered with the Creative Commons attribution license, which allows third parties to use the published information whenever they mention the authorship of the work and the First publication in this journal.
b. Authors may make other independent and additional contractual arrangements for non-exclusive distribution of the version of the article published in this journal (eg, include it in an institutional repository or publish it in a book) as long as it clearly indicates that the work Was first published in this journal.
c. Authors are encouraged to publish their work on the Internet (for example, on institutional or personal pages) before and during the review and publication process, as it can lead to productive exchanges and a greater and faster dissemination of work Published (see The Effect of Open Access).




