Pitahaya (Hylocereus guatemalensis) explants from areoles: Protocol for in vitro regeneration and successful acclimatization
DOI:
https://doi.org/10.17268/sci.agropecu.2025.033Keywords:
Areola, cactus, in vitro clonal propagation, disinfection, culture media, growth regulators, rootingAbstract
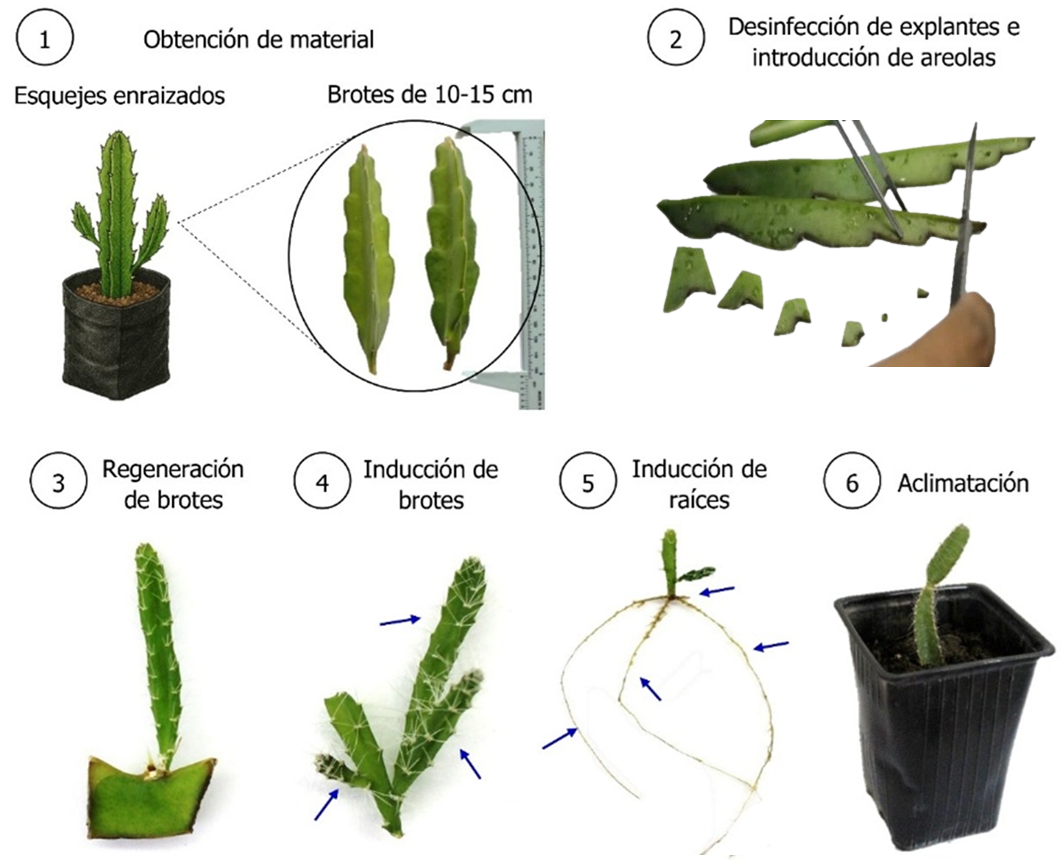
In vitro micropropagation of pitahaya has been presented as an alternative for obtaining high quality plant material and mass production in reduced and controlled spaces. The present study aimed to achieve in vitro regeneration of shoots and roots of H. guatemalensis from areoles and their survival during ex vitro acclimatization. Different treatments with culture media, growth regulators and substrates were evaluated to obtain plants with normal development. Shoot regeneration from areoles was achieved 14 days, enriching the Murashige and Skoog (MS) medium with different concentrations of benzylaminopurine (BAP). The induction of shoots without callus formation and with greater shoot length and diameter was achieved using Woody Plant Medium (WPM) without cytokinins. On the other hand, root induction began at 14 days, obtaining between 90 and 100% of rooted explants, with the highest number of roots produced in media supplemented with indolbutyric acid (IBA), and the greatest lengths with MS medium without regulators. 100% survival was achieved using a mixture of perlite and peat in a 1:1 ratio, as well as higher values of cladode length and diameter. This study reports, for the first time, the protocols for in vitro regeneration of pitahaya H. guatemalensis explants from areoles, as well as their successful acclimatization.
References
Amorim, T. A., de Brito Araujo Carvalho, A. J., da Silva Figueiredo, L., Lima, M. dos S., Sarinho, A. M., Santos, N. C., Lisboa, H. M., Souza Gusmão, T. A., & de Pereira Gusmão, R. (2025). Structure-function relationship and antioxidant mechanisms of pectin from red and white pitaya peels for functional food applications. Food Hydrocolloids, 167. https://doi.org/10.1016/j.foodhyd.2025.111455
Bouzroud, S., El Maaiden, E., Sobeh, M., Devkota, K. P., Boukcim, H., Kouisni, L., & El Kharrassi, Y. (2022). Micropropagation of Opuntia and Other Cacti Species Through Axillary Shoot Proliferation: A Comprehensive Review. Front. Plant Sci., 13. https://doi.org/10.3389/fpls.2022.926653
Bozkurt, T., İnan, S., & Dündar, İ. (2020). Micropropagation of Different Pitaya Varieties. International Journal of Agricultural and Natural Sciences, 13(1), 39–46. https://ijans.org/index.php/ijans/article/view/496/474
Bozkurt, T., İnan, S., Dündar, İ., & Özdemir, S. K. (2022). Effect of Different Plant Growth Regulators on Micropropagation of Some Pitaya Varieties. Journal of Tropical Life Science, 12(2), 183–190. https://doi.org/10.11594/jtls.12.02.04
Chhetri, S., Hasan, M. A., & Tamang, A. (2021). Influence of varying length of stem cutting and IBA concentrations on root and shoot growth in Dragon Fruit cv Giant White (Hylocereus undatus). Environment and Ecology, 39(4A), 1114–1118. https://www.researchgate.net/publication/359857530
Chongloi, L., Gunnaiah, R., Hipparagi, K., Guranna, P., Prakasha, D. P., Chittapur, R., & Kamble, A. (2023). Optimization of low-cost potting mixture for hardening of in vitro raised plants of dragon fruit. Journal of Applied Horticulture, 25(3), 286–290. https://doi.org/10.37855/jah.2023.v25i03.51
Cortés-Olmos, C., Guerra-Sandoval, V. M., Guijarro-Real, C., Pineda, B., Fita, A., & Rodríguez-Burruezo, A. (2025). Response to In Vitro Micropropagation of Plants with Different Degrees of Variegation of the Commercial Gymnocalycium cv. Fancy (Cactaceae). Plants, 14(7). https://doi.org/10.3390/plants14071091
Dewir, Y. H., Habib, M. M., Alaizari, A. A., Malik, J. A., Al-Ali, A. M., Al-Qarawi, A. A. A., & Alwahibi, M. S. (2023). Promising Application of Automated Liquid Culture System and Arbuscular Mycorrhizal Fungi for Large-Scale Micropropagation of Red Dragon Fruit. Plants, 12(5), 1037. https://doi.org/10.3390/plants12051037
Espinosa-Leal, C. A., Puente-Garza, C. A., & García-Lara, S. (2018). In vitro plant tissue culture: means for production of biological active compounds. Planta, 248, 1–18. https://doi.org/10.1007/s00425-018-2910-1
Fan, Q. J., Zheng, S. C., Yan, F. X., Zhang, B. X., Qiao, G., & Wen, X. P. (2013). Efficient regeneration of dragon fruit (Hylocereus undatus) and an assessment of the genetic fidelity of in vitro-derived plants using ISSR markers. Journal of Horticultural Science and Biotechnology, 88(5), 631–637. https://doi.org/10.1080/14620316.2013.11513017
Gauer, P. C., Ferreira, A. C. V., Siqueira, M. S., Gimênes-Junior, H., Abe, H. A., Fantini-Hoag, L., Godoy, A. C., & Honorato, C. A. (2025). Hydroalcoholic extract of pitaya (Hylocereus guatemalensis) supplied in the diet for blue Betta splendens and its effects on fish pigmentation. Anais Da Academia Brasileira de Ciencias, 97(2). https://doi.org/10.1590/0001-3765202520240212
Hua, Q., Chen, P., Liu, W., Ma, Y., Liang, R., Wang, L., Wang, Z., Hu, G., & Qin, Y. (2015). A protocol for rapid in vitro propagation of genetically diverse pitaya. Plant Cell Tiss Organ Cult, 120, 741–745. https://doi.org/10.1007/s11240-014-0643-9
Lee, Y. C., & Chang, J. C. (2022). Development of an Improved Micropropagation Protocol for Red-Fleshed Pitaya ‘Da Hong’ with and without Activated Charcoal and Plant Growth Regulator Combinations. Horticulturae, 8(2), 104. https://doi.org/10.3390/horticulturae8020104
Liaotrakoon, W. (2013). Characterization of dragon fruit (Hylocereus spp.) components with valorization potential [PhD thesis]. Ghent University.
Malda, G., Backhaus, R. A., & Martin, C. (1999). Alterations in growth and crassulacean acid metabolism (CAM) activity of in vitro cultured cactus. Plant Cell, Tissue and Organ Culture, 58, 1–9. https://doi.org/https://doi.org/10.1023/A:1006377206855
Marcotrigiano, M., McGlew, S. E., Hackett, G., & Chawla, B. (1996). Shoot regeneration from tissue-cultured leaves of the American cranberry (Vaccinium macrocarpon). Plant Cell, Tissue and Organ Culture, 44, 195–199. https://doi.org/https://doi.org/10.1007/BF00048524
Martínez Arroyo, M. C., Mancilla-Álvarez, E., Spinoso-Castillo, J. L., & Bello-Bello, J. J. (2023). Evaluation of the effect of different culture systems on photomixotrophic capacity during in vitro multiplication of pitahaya (Hylocereus undatus). South African Journal of Botany, 159, 396–404. https://doi.org/10.1016/j.sajb.2023.06.013
Menezes, T. P., Gomes, W. de A., Pio, L. A. S., Pasqual, M., & Ramos, J. D. (2012). Micropropagação e endorreduplicação em pitaya vermelha, Hylocereus undatus HAW. Bioscience Journal, 28(6), 868–876.
Mercado-Silva, E. M. (2018). Pitaya— Hylocereus undatus (Haw). Exotic Fruits, 339–349. https://doi.org/10.1016/b978-0-12-803138-4.00045-9
Mohamed-Yasseen, Y. (2002). Micropropagation of pitaya (Hylocereus undatus Britton et Rose). In Vitro Cellular and Developmental Biology - Plant, 38(5), 427–429. https://doi.org/10.1079/IVP2002312
Navarro-Sandoval, B. G., & Canales-Carrera, E. E. (2021). Efecto de diferentes concentraciones de benzilaminopurina (BAP) sobre el establecimiento in vitro de pitahaya (Hylocereus guatemalensis). Revista De Innovación Y Transferencia Productiva, 2(1), e002. https://doi.org/10.54353/ritp.v2i1.e002
Phillips, G. C., & Garda, M. (2019). Plant tissue culture media and practices: an overview. In Vitro Cellular and Developmental Biology - Plant, 55(3), 242–257. https://doi.org/10.1007/s11627-019-09983-5
Qin, J., Wang, Y., He, G., Chen, L., He, H., Cheng, X., Xu, K., & Zhang, D. (2017). High-efficiency micropropagation of dormant buds in spine base of red pitaya (Hylocereus polyrhizus) for industrial breeding. International Journal of Agriculture and Biology, 19(1), 193–198. https://doi.org/10.17957/IJAB/15.0264
Rodrigues, M. A., da Silveira, F. A., Moreira, R. A., Pádua, M. S., Pinto, J. E. B. P., Pio, L. A. S., dos Santos, D. N., de Sousa Bueno Filho, J. S., & Reis, L. A. C. (2022). Regeneration of pitaya by indirect organogenesis evaluated by scanning electron microscopy and flow cytometry. Pesquisa Agropecuaria Brasileira, 57. https://doi.org/10.1590/S1678-3921.pab2022.v57.02312
Salas, L., Foroughbackch, R., Díaz, M., Ávila, M., & Flores, A. (2011). Germinación in vitro de cactáceas, utilizando zeolita como sustrato alternativo. Revista Mexicana de Ciencias Agrícolas, 3, 565–575.
Shah, K., Chen, J., Chen, J., & Qin, Y. (2023). Pitaya Nutrition, Biology, and Biotechnology: A Review. Int. J. Mol. Sci., 24. https://doi.org/10.3390/ijms241813986
Trivellini, A., Lucchesini, M., Ferrante, A., Massa, D., Orlando, M., Incrocci, L., & Mensuali-Sodi, A. (2020). Pitaya, an attractive alternative crop for mediterranean region. Agronomy, 10(8). https://doi.org/10.3390/agronomy10081065
Vargas Gutiérrez, K., & López Montañez, R. N. (2020). Guía técnica del cultivo de pitahaya (Hylocereus megalanthus) en la región Amazonas (Instituto Nacional de Innovación Agraria (INIA), Ed.).
Verona-Ruiz, A., Urcia-Cerna, J., & Paucar-Menacho, L. M. (2020). Pitahaya (Hylocereus spp.): Cultivo, características fisicoquímicas, composición nutricional y compuestos bioactivos. Scientia Agropecuaria, 11(3), 439–453. https://doi.org/10.17268/sci.agropecu.2020.03.16
Viñas, M., Fernández-Brenes, M., Azofeifa, A., & Jiménez, V. M. (2012). In vitro propagation of purple pitahaya (Hylocereus costaricensis [F.A.C. Weber] Britton & Rose) cv. Cebra. In Vitro Cell.Dev.Biol.-Plant, 48(5), 469–477. https://doi.org/10.1007/s11627-012-9439-y
Wan Anuar, W. N. H., Tan, S. H., Shiekh Mahmud, S. H. R., Mior Norazmi, W. N. B., & Engku Safruddin, E. F. S. (2019). Enhancement effect of BAP and spent mushroom compost in micro-propagation of Sabah snake grass. American Journal of Biochemistry and Biotechnology, 15(4), 190–197. https://doi.org/10.3844/ajbbsp.2019.190.197
Yasmin, A., Sumi, M. J., Akter, K., Rabbi, R. H. M., Almoallim, H. S., Ansari, M. J., Hossain, A., & Imran, S. (2024). Comparative analysis of nutrient composition and antioxidant activity in three dragon fruit cultivars. PeerJ, 12(7). https://doi.org/10.7717/peerj.17719
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Scientia Agropecuaria

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
The authors who publish in this journal accept the following conditions:
a. The authors retain the copyright and assign to the magazine the right of the first publication, with the work registered with the Creative Commons attribution license, which allows third parties to use the published information whenever they mention the authorship of the work and the First publication in this journal.
b. Authors may make other independent and additional contractual arrangements for non-exclusive distribution of the version of the article published in this journal (eg, include it in an institutional repository or publish it in a book) as long as it clearly indicates that the work Was first published in this journal.
c. Authors are encouraged to publish their work on the Internet (for example, on institutional or personal pages) before and during the review and publication process, as it can lead to productive exchanges and a greater and faster dissemination of work Published (see The Effect of Open Access).




