Nematophagous fungi for integrated management of Meloidogyne (Tylenchida): a review of taxonomic diversity, mechanisms of action and potential as biological control agents
DOI:
https://doi.org/10.17268/sci.agropecu.2025.041Keywords:
Agricultural pests, Arthrobotrys, egg parasites, nematophagous fungi, plant parasites, root-knot nematodesAbstract
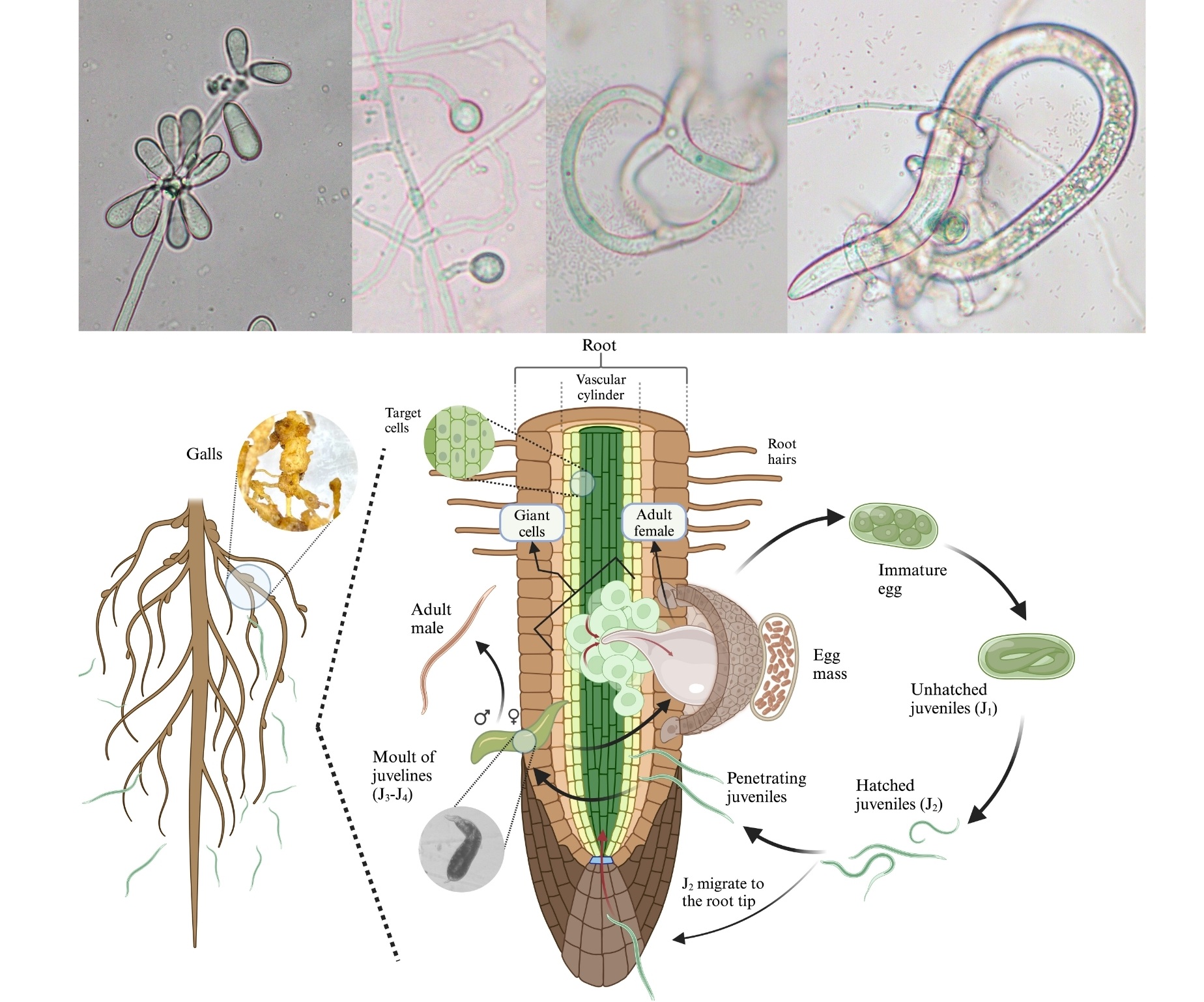
Root-knot nematodes (RKNs) are classified under the genus Meloidogyne and are among the most devastating pests affecting strategical agricultural crops. They attack a wide variety of plant species, including vegetables, fruit trees and ornamental plants, causing root deformities and even lead to plant death in severe cases of infestation. These nematodes contribute to substantial crop yield loss and affect the quality of harvested products. Although synthetic nematicides are available for the control of these pest organisms, there is a growing emphasis on exploring sustainable and eco-friendly alternatives, such as nematophagous fungi like the genera Purpureocillium, Arthrobotrys, Dactylellina, Orbilia, and Trichoderma, among others. Here a review of literature on the matter is given, with a focus on the taxonomic classification of the most relevant fungal orders and genera, their diagnostic features, mechanisms of action, and potential as biological control agents (BCAs) against Meloidogyne species. Other relevant aspects addressed in this review include a brief description of the nematode genus Meloidogyne, along with the symptoms it causes in host plants, such as root gall formation, stunted growth, and yellowing of foliage, among others. It also describes integrated pest management (IPM) strategies such as crop rotation, resistant crops, soil solarization, trap crops, as well as currently used chemical control techniques. Biological control alternatives are also presented with particular emphasis on nematophagous fungi. Future research should focus on improving the formulae of biological agents based on nematophagous fungi under field conditions and understanding their ecological roles and interactions in soil microbiomes.
References
Afzal, A., & Mukhtar, T. (2024). Revolutionizing nematode management to achieve global food security goals - an overview. Heliyon, 10(3). https://doi.org/10.1016/j.heliyon.2024.e25325
Ali, M. A., Azeem, F., Abbas, A., Joyia, F. A., Li, H., & Dababat, A. A. (2017). Transgenic strategies for enhancement of nematode resistance in plants. Frontiers in Plant Science, 8. https://doi.org/10.3389/fpls.2017.00750
Ali, N., Chapuis, E., Tavoillot, J., & Mateille, T. (2014). Plant-parasitic nematodes associated with olive tree (Olea europaea L.) with a focus on the Mediterranean Basin: a review. Comptes Rendus - Biologies, 337(7–8), 423–442. https://doi.org/10.1016/j.crvi.2014.05.006
Amatuzzi, R. F., Poitevin, C. G., Poltronieri, A. S., Zawadneak, M. A. C., & Pimentel, I. C. (2018). Susceptibility of Duponchelia fovealis Zeller (Lepidoptera: Crambidae) to soil-borne entomopathogenic fungi. Insects, 9(2), 70. https://doi.org/10.3390/insects9020070
Aminuzzaman, F. M., Xie, H. Y., Duan, W. J., Sun, B. D., & Liu, X. Z. (2013). Isolation of nematophagous fungi from eggs and females of Meloidogyne spp. and evaluation of their biological control potential. Biocontrol Science and Technology, 23(2), 170–182. https://doi.org/10.1080/09583157.2012.745484
Azeem, W., Mukhtar, T., & Hamid, T. (2020). Evaluation of Trichoderma harzianum and Azadirachta indica in the management of Meloidogyne incognita in tomato. Pakistan Journal of Zoology, 53(1). https://doi.org/10.17582/journal.pjz/20190905100940
Azlay, L., El Boukhari, M. E. M., Mayad, E. H., & Barakate, M. (2023). Biological management of root-knot nematodes (Meloidogyne spp.): a review. Organic Agriculture, 13(1). https://doi.org/10.1007/s13165-022-00417-y
Bakr, R. A., Mahdy, M. E., & Mousa, M. E. (2013). Efficacy of soil solarization and post-planting mulch on control of root-knot nematodes. Pakistan Journal of Nematology, 31, 71–76.
Barron, G. L. (2004). Fungal parasites and predators of rotifers, nematodes, and other invertebrates. In G. M. Muller, G. F. Bills, & M. S. Foster (Eds.), Biodiversity of Fungi (pp. 435–450). Elsevier. https://doi.org/10.1016/B978-012509551-8/50022-2
Bontempo, A. F., Fernandes, R. H., Lopes, J., Freitas, L. G., & Lopes, E. A. (2014). Pochonia chlamydosporia controls Meloidogyne incognita on carrot. Australasian Plant Pathology, 43(4), 421–424. https://doi.org/10.1007/s13313-014-0283-x
Borah, B., Ahmed, R., Hussain, M., Phukon, P., Wann, S. B., Sarmah, D. K., & Bhau, B. S. (2018). Suppression of root-knot disease in Pogostemon cablin caused by Meloidogyne incognita in a rhizobacteria mediated activation of phenylpropanoid pathway. Biological Control, 119, 43–50. https://doi.org/10.1016/j.biocontrol.2018.01.003
Candido, V., D’Addabbo, T., Basile, M., Castronuovo, D., & Miccolis, V. (2008). Greenhouse soil solarization: effect on weeds, nematodes and yield of tomato and melon. Agronomy for Sustainable Development, 28(2), 221–230. https://doi.org/10.1051/agro:2007053
Castagnone-Sereno, P., Danchin, E. G. J., Perfus-Barbeoch, L., & Abad, P. (2013). Diversity and evolution of root-knot nematodes, genus Meloidogyne: new insights from the genomic era. Annual Review of Phytopathology, 51, 203–220. https://doi.org/10.1146/annurev-phyto-082712-102300
Cayrol, J. C., Castet, R., & Samson, R. A. (1986). Comparative activity of different Hirsutella species towards three plant parasitic nematodes. Revue Nématologie, 9(4), 412–414.
Chen, P., & Tsay, T. T. (2006). Effect of crop rotation on Meloidogyne spp. and Pratylenchus spp. populations in strawberry fields in Taiwan. Journal of Nematology, 38(3), 339–344.
Collange, B., Navarrete, M., Peyre, G., Mateille, T., & Tchamitchian, M. (2011). Root-knot nematode (Meloidogyne) management in vegetable crop production: the challenge of an agronomic system analysis. Crop Protection, 30(10), 1251–1262. https://doi.org/10.1016/j.cropro.2011.04.016
Dai, Z., Gan, Y., Zhao, P., & Li, G. (2022). Secondary metabolites from the endoparasitic nematophagous fungus Harposporium anguillulae YMF 1.01751. Microorganisms, 10(8), 1553. https://doi.org/10.3390/microorganisms10081553
De Lisser, L. (2022). Evaluación del control de namatodos fitoparásitos en el cultivo de arroz (Oryza sativa L.), utilizando el agente de control biológico Pochonia chlamydosporia (Goddard) Zare Y Gams [Universidad de Panamá]. https://up-rid.up.ac.pa/6478/
Decraemer, W., & Hunt, D. J. (2013). Structure and classification. In R. N. Perry & M. Moens (Eds.), Plant Nematology (2nd ed.). https://doi.org/10.1525/9780520906136-005
Degenkolb, T., & Vilcinskas, A. (2016). Metabolites from nematophagous fungi and nematicidal natural products from fungi as an alternative for biological control. Part I: metabolites from nematophagous ascomycetes. Applied Microbiology and Biotechnology, 100(9), 3799–3812. https://doi.org/10.1007/s00253-015-7233-6
Devi, G. (2018). Utilization of nematode destroying fungi for management of plant-parasitic nematodes- a review. Biosciences, Biotechnology Research Asia, 15(2). https://doi.org/10.13005/bbra/2642
Drechsler, C. (1936). A new species of Stylopage preying on nematodes. Mycologia, 28(3), 241–246. https://doi.org/10.1080/00275514.1936.12017136
Drechsler, C. (1941). Four phycomycetes destructive to nematodes and rhizopods. Mycologia, 33(3), 248–269. https://doi.org/10.1080/00275514.1941.12020814
Duarte, R. T., Gonçalves, K. C., Espinosa, D. J. L., Moreira, L. F., De Bortoli, S. A., Humber, R. A., & Polanczyk, R. A. (2016). Potential of entomopathogenic fungi as biological control agents of Diamondback moth (Lepidoptera: Plutellidae) and compatibility with chemical insecticides. Journal of Economic Entomology, 109(2), 594–601. https://doi.org/10.1093/jee/tow008
Dürschner-Pelz, U. U. (1987). Traps of Nematoctonus leiosporus—an unusual feature of an endoparasitic nematophagous fungus. Transactions of the British Mycological Society, 88(1), 129–130. https://doi.org/10.1016/S0007-1536(87)80198-5
El-Borai, F. E., Campos-Herrera, R., Stuart, R. J., & Duncan, L. W. (2011). Substrate modulation, group effects and the behavioral responses of entomopathogenic nematodes to nematophagous fungi. Journal of Invertebrate Pathology, 106(3), 347–356. https://doi.org/10.1016/j.jip.2010.12.001
Elkhateeb, W. A., Elghwas, D. E., & Daba, G. M. (2023). Nematophagous fungi as an extraordinary tool to control parasitic nematodes: a review. Environmental Science Archives, 2(1), 52–58. https://doi.org/10.5281/zenodo.7540410
Elling, A. A. (2013). Major emerging problems with minor Meloidogyne species. Phytopathology, 103(11). https://doi.org/10.1094/PHYTO-01-13-0019-RVW
Erazo Sandoval, N. S., Echeverría Guadalupe, M. M., Jave Nakayo, J. L., León Reyes, H. A., Lindao Córdova, V. A., Manzano Ocaña, J. C., & Inca Chunata, N. M. (2020). Effect of Pleurotus ostreatus (Jacq.) and Trichoderma harzianum (Rifai) on Meloidogyne incognita (Kofoid & White) in tomato (Solanum lycopersicum Mill.). Acta Scientiarum. Biological Sciences, 42, e47522. https://doi.org/10.4025/actascibiolsci.v42i1.47522
Everts, K. L., Sardanelli, S., Kratochvil, R. J., Armentrout, D. K., & Gallagher, L. E. (2006). Root-knot and root-lesion nematode suppression by cover crops, poultry litter, and poultry litter compost. Plant Disease, 90(4), 487–492. https://doi.org/10.1094/PD-90-0487
Fairbairn, D. J., Cavallaro, A. S., Bernard, M., Mahalinga-Iyer, J., Graham, M. W., & Botella, J. R. (2007). Host-delivered RNAi: an effective strategy to silence genes in plant parasitic nematodes. Planta, 226(6), 1525–1533. https://doi.org/10.1007/s00425-007-0588-x
Fuller, V. L., Lilley, C. J., & Urwin, P. E. (2008). Nematode resistance. New Phytologist, 180(1), 27–44. https://doi.org/10.1111/j.1469-8137.2008.02508.x
Gams, W., & Zare, R. (2003). A taxonomic review of the clavicipitaceous anamorphs parasitizing nematodes and other microinvertebrates. In J. F. Jr. White, C. W. Bacon, N. L. Hywel-Jones, & J. W. Spatafora (Eds.), Clavicipitalean Fungi: Evolutionary Biology, Chemistry, Biocontrol, and Cultural Impacts (Vol. 19, pp. 17–73). Marcel-Dekker. https://doi.org/10.1201/9780203912706.pt1
Gaur H.S., & Dhingra A. (1991). Management of Meloidogyne incognita and Rotylenchulus reniformis in nursery-beds by soil solarization and organic soil amendment. Revue de Nématologie, 14(2), 189–195.
Grabau, Z. J., Liu, C., & Sandoval-Ruiz, R. (2021). Meloidogyne incognita management by nematicides in tomato production. Journal of Nematology, 53(1), 1–12. https://doi.org/10.21307/jofnem-2021-055
Gray, N. F. (1987). Nematophagous fungi with particular reference to their ecology. Biological Reviews - Cambridge Philosophical Society, 62(3). https://doi.org/10.1111/j.1469-185X.1987.tb00665.x
Grover, M., Fasseas, M. K., Essmann, C., Liu, K., Braendle, C., Félix, M.-A., Glockling, S. L., & Barkoulas, M. (2021). Infection of C. elegans by Haptoglossa species reveals shared features in the host response to oomycete detection. Frontiers in Cellular and Infection Microbiology, 11. https://doi.org/10.3389/fcimb.2021.733094
Hajji-Hedfi, L., Larayedh, A., Tormo, L., Regaieg, H., & Horrigue-Raouani, N. (2018). Isolation and characterization of Lecanicillium sp. for antagonistic activity against Meloidogyne javanica. In A. Kallel, M. Ksibi, H. Ben Dhia, & N. Khélifi (Eds.), Recent Advances in Environmental Science from the Euro-Mediterranean and Surrounding Regions (pp. 395–398). Springer, Cham. https://doi.org/10.1007/978-3-319-70548-4_124
Halford, P. D., Russell, M. D., & Evans, K. (1999). Use of resistant and susceptible potato cultivars in the trap cropping of potato cyst nematodes, Globodera pallida and G. rostochiensis. Annals of Applied Biology, 134(3), 321–327. https://doi.org/10.1111/j.1744-7348.1999.tb05271.x
Howland, A., & Quintanilla, M. (2023). Plant-parasitic nematodes and their effects on ornamental plants: a review. Journal of Nematology, 55(1). https://doi.org/10.2478/jofnem-2023-0007
Huang, W. K., Cui, J. K., Liu, S. M., Kong, L. A., Wu, Q. S., Peng, H., He, W. T., Sun, J. H., & Peng, D. L. (2016). Testing various biocontrol agents against the root-knot nematode (Meloidogyne incognita) in cucumber plants identifies a combination of Syncephalastrum racemosum and Paecilomyces lilacinus as being most effective. Biological Control, 92, 31–37. https://doi.org/10.1016/j.biocontrol.2015.09.008
Jansson, H. B., Jeyaprakash, A., & Zuckerman, B. M. (1985). Control of root-knot nematodes on tomato by the endoparasitic fungus Meria coniospora. Journal of Nematology, 17(3), 327–329.
Jones, J. T., Haegeman, A., Danchin, E. G. J., Gaur, H. S., Helder, J., Jones, M. G. K., Kikuchi, T., Manzanilla-López, R., Palomares-Rius, J. E., Wesemael, W. M. L., & Perry, R. N. (2013). Top 10 plant-parasitic nematodes in molecular plant pathology. In Molecular Plant Pathology (Vol. 14, Issue 9, pp. 946–961). https://doi.org/10.1111/mpp.12057
Jones, M. G. K., & Goto, D. B. (2011). Root-knot nematodes and giant cells. In J. Jones, C. Fenoll, & G. Gheysen (Eds.), Genomics and Molecular Genetics of Plant-Nematode Interactions (pp. 83–100). Springer Netherlands. https://doi.org/10.1007/978-94-007-0434-3_5
Kennedy, N., & Tampion, J. (1978). A nematotoxin from Nematoctonus robustus. Transactions of the British Mycological Society, 70(1), 140–141. https://doi.org/10.1016/S0007-1536(78)80184-3
Kerry, B. R., & Crump, D. H. (1980). Two fungi parasitic on females of cystnematodes (Heterodera spp.). Transactions of the British Mycological Society, 74(1), 119–125. https://doi.org/10.1016/S0007-1536(80)80017-9
Khan, A., Ahmad, G., Haris, M., & Khan, A. A. (2022). Bio-organics management: novel strategies to manage root-knot nematode, Meloidogyne incognita pest of vegetable crops. Gesunde Pflanzen, 75(1), 193–209. https://doi.org/10.1007/S10343-022-00679-2
Khan, A., Haris, M., Hussain, T., Khan, A. A., Laasli, S.-E., Lahlali, R., & Mokrini, F. (2023). Counter-attack of biocontrol agents: environmentally benign approaches against root-knot nematodes (Meloidogyne spp.) on agricultural crops. Heliyon, 9(11), e21653. https://doi.org/10.1016/j.heliyon.2023.e21653
Khanal, C., & Desaeger, J. A. (2020). On-farm evaluations of non-fumigant nematicides on cucurbits. Crop Protection, 133, 105152. https://doi.org/10.1016/j.cropro.2020.105152
Khanal, C., Harshman, D., & Giles, C. (2022). On-farm evaluations of nonfumigant nematicides on nematode communities of peach. Phytopathology, 112(10), 2218–2223. https://doi.org/10.1094/PHYTO-04-22-0122-R
Kratochvil, R. J., Sardanelli, S., Everts, K., & Gallagher, E. (2004). Evaluation of crop rotation and other cultural practices for management of root‐knot and lesion nematodes. Agronomy Journal, 96(5), 1419–1428. https://doi.org/10.2134/agronj2004.1419
Kudrin, A. A., Tsurikov, S. M., & Tiunov, A. V. (2015). Trophic position of microbivorous and predatory soil nematodes in a boreal forest as indicated by stable isotope analysis. Soil Biology and Biochemistry, 86, 193–200. https://doi.org/10.1016/j.soilbio.2015.03.017
Kumar, D. (2024). Effectiveness of various nematode-trapping fungi for biocontrol of the Meloidogyne incognita in tomato (Lycopersicion esculentum Mill.). Rhizosphere, 29, 100845. https://doi.org/10.1016/J.RHISPH.2023.100845
Kumar, K. K. (2020). Fungi: a bio-resource for the control of plant parasitic nematodes. In A. Yadav, S. Mishra, D. Kour, N. Yadav, & A. Kumar (Eds.), Agriculturally important fungi for sustainable agriculture (Vol. 2, pp. 285–311). Springer Nature. https://doi.org/10.1007/978-3-030-48474-3_10
Kumar, N., & Singh, K. P. (2011). Use of Dactylaria brochopaga, a predacious fungus, for managing root-knot disease of wheat (Triticum aestivum) caused by Meloidogyne graminicola. Mycobiology, 39(2), 113. https://doi.org/10.4489/MYCO.2011.39.2.113
Kumari, D., Yadav, N. K., Singh, N., Saran, M. K., & Rahul. (2023). Characterization and evaluation of native Trichoderma isolates for antagonistic activity against Fusarium oxysporum f. sp. ciceris. Plant Disease Research, 38(2), 140–147. https://doi.org/10.5958/2249-8788.2023.00017.9
Kundu, A., Saha, S., Walia, S., & Dutta, T. K. (2016). Anti-nemic secondary metabolites produced by Fusarium oxysporum f. sp. ciceris. Journal of Asia-Pacific Entomology, 19(3), 631–636. https://doi.org/10.1016/j.aspen.2016.06.003
Kwok, O. C. H., Plattner, R., Weisleder, D., & Wicklow, D. T. (1992). A nematicidal toxin from Pleurotus ostreatus NRRL 3526. Journal of Chemical Ecology, 18(2), 127–136. https://doi.org/10.1007/BF00993748
Lamovšek, J., Urek, G., & Trdan, S. (2013). Biological control of root-knot nematodes (Meloidogyne spp.): microbes against the pests. Acta Agriculturae Slovenica, 101(2), 263–275. https://doi.org/10.2478/acas-2013-0022
Li, G.-H., & Zhang, K.-Q. (2014). Nematode-toxic fungi and their nematicidal metabolites. In K. Zhang & K. Hyde (Eds.), Nematode-Trapping Fungi (Vol. 23). Springer, Dordrecht. https://doi.org/10.1007/978-94-017-8730-7_7
Liang, L. M., Zou, C. G., Xu, J., & Zhang, K. Q. (2019). Signal pathways involved in microbe-nematode interactions provide new insights into the biocontrol of plant-parasitic nematodes. Philosophical Transactions of the Royal Society B: Biological Sciences, 374(1767). https://doi.org/10.1098/rstb.2018.0317
Liang, L., Meng, Z., Ye, F., Yang, J., Liu, S., Sun, Y., Guo, Y., Mi, Q., Huang, X., Zou, C., Rao, Z., Lou, Z., & Zhang, K. (2010). The crystal structures of two cuticle–degrading proteases from nematophagous fungi and their contribution to infection against nematodes. The FASEB Journal, 24(5), 1391–1400. https://doi.org/10.1096/fj.09-136408
Lilley, C. J., Kyndt, T., & Gheysen, G. (2011). Nematode resistant GM crops in industrialised and developing countries. In J. Jones, G. Gheysen, & C. Carmen Fenoll (Eds.), Genomics and Molecular Genetics of Plant-Nematode Interactions (Springer, pp. 517–541). Science+Business Media. https://doi.org/10.1007/978-94-007-0434-3
Liu, C., & Grabau, Z. (2022). Meloidogyne incognita management using fumigant and non-fumigant nematicides on sweet potato. Journal of Nematology, 54(1). https://doi.org/10.2478/jofnem-2022-0026
Liu, X., Xiang, M., & Che, Y. (2009). The living strategy of nematophagous fungi. Mycoscience, 50(1), 20–25. https://doi.org/10.1007/s10267-008-0451-3
Lopes, E. A., Dallemole-Giaretta, R., dos Santos Neves, W., Parreira, D. F., & Ferreira, P. A. (2019). Eco-friendly approaches to the management of plant-parasitic nematodes. In R. Ansari & I. Mahmood (Eds.), Plant Health Under Biotic Stress (pp. 167–186). Springer. https://doi.org/10.1007/978-981-13-6043-5_9
López-Llorca, L. V., Maciá-Vicente, J. G., & Jansson, H.-B. (2008). Mode of action and interactions of nematophagous fungi. In A. Ciancio & K. G. Mukerji (Eds.), Integrated management and biocontrol of vegetable and grain crops nematodes (Vol. 2, 51–76). Springer. https://doi.org/10.1007/978-1-4020-6063-2_3
Luo, H., Liu, Y., Fang, L., Li, X., Tang, N., & Zhang, K. (2007). Coprinus comatus damages nematode cuticles mechanically with spiny balls and produces potent toxins to immobilize nematodes. Applied and Environmental Microbiology, 73(12), 3916–3923. https://doi.org/10.1128/AEM.02770-06
Luo, H., Mo, M., Huang, X., Li, X., & Zhang, K. (2004). Coprinus comatus: a basidiomycete fungus forms novel spiny structures and infects nematode. Mycologia, 96(6), 1218. https://doi.org/10.2307/3762137
Maulana, I., Lubis, S. S., Harahap, D., Arskadius, N. U., & Concepcion, R. S. (2024). Antagonistic activity of Trichoderma sp. against pathogens in the leaves of Allium ascalonicum L. Narra X, 2(1). https://doi.org/10.52225/narrax.v2i1.125
Messa, V. R., Torres da Costa, A. C., Kuhn, O. J., & Stroze, C. T. (2020). Nematophagous and endomycorrhizal fungi in the control of Meloidogyne incognita in soybean. Rhizosphere, 15. https://doi.org/10.1016/j.rhisph.2020.100222
Moosavi, M. R. (2020). Efficacy of microbial biocontrol agents in integration with other managing methods against phytoparasitic nematodes. In R. Ansari, R. Rizvi, & I. Mahmood (Eds.), Management of Phytonematodes: Recent Advances and Future Challenges (pp. 229–258). Springer. https://doi.org/10.1007/978-981-15-4087-5_10
Moosavi, M. R., & Zare, R. (2020). Fungi as biological control agents of plant-parasitic nematodes. In J.-M. Mérillon & K. G. Ramawat (Eds.), Plant Defence: Biological Control (Vol. 2, pp. 333–384). Springer Nature. https://doi.org/10.1007/978-3-030-51034-3_14
Nandeesha, C. (2020). In vitro bioassay of Meloidogyne incognita juveniles against biocontrol agents. Journal of Entomology and Zoology Studies, 8(4), 338–340.
Nekoval, S. N., Churikova, A. K., Chernyakovich, M. N., & Pridannikov, M. V. (2023). Primary screening of microorganisms against Meloidogyne hapla (Chitwood, 1949) under the conditions of laboratory and vegetative tests on tomato. Plants, 12(18), 3323. https://doi.org/10.3390/plants12183323
Nordbring-Hertz, B. (2004). Morphogenesis in the nematode-trapping fungus Arthrobotrys oligospora – an extensive plasticity of infection structures. Mycologist, 18(3), 125–133. https://doi.org/10.1017/S0269915XO4003052
Nordbring‐Hertz, B., Jansson, H., & Tunlid, A. (2006). Nematophagous fungi. Encyclopedia of Life Sciences. https://doi.org/10.1038/npg.els.0004293
Nyangwire, B., Ocimati, W., Tazuba, A. F., Blomme, G., Alumai, A., & Onyilo, F. (2024). Pleurotus ostreatus is a potential biological control agent of root-knot nematodes in eggplant (Solanum melongena). Frontiers in Agronomy, 6. https://doi.org/10.3389/fagro.2024.1464111
Onkendi, E. M., Kariuki, G. M., Marais, M., & Moleleki, L. N. (2014). The threat of root-knot nematodes (Meloidogyne spp.) in Africa: a review. Plant Pathology, 63(4), 727–737. https://doi.org/10.1111/ppa.12202
Palomares-Rius, J. E., Hasegawa, K., Siddique, S., & Vicente, C. S. L. (2021). Editorial: protecting our crops - approaches for plant parasitic nematode control. Frontiers in Plant Science, 12. https://doi.org/10.3389/fpls.2021.726057
Peraza Padilla, W., Orozco Aceves, M., & Esquivel Hernández, A. (2014). Evaluación in vitro de hongos nematófagos en zonas arroceras de Costa Rica contra el nematodo agallador Meloidogyne javanica. Agronomía Costarricense, 38(2). https://doi.org/10.15517/rac.v38i2.17271
Pérez-González, J. O., Ramírez-Rojas, S. G., Rocha-Rodríguez, R., Ornelas-Ocampo, K., Vázquez-Alvarado, J. M. P., Hernández-Guzmán, F. J., & Garduño-Audelo, M. (2023). In vitro antagonism of Trichoderma against Rhizoctonia solani. Revista Mexicana de Fitopatología, Mexican Journal of Phytopathology, 41(3). https://doi.org/10.18781/R.MEX.FIT.2304-2
Priya, D. B., Somasekhar, N., Prasad, J. S., & Kirti, P. B. (2011). Transgenic tobacco plants constitutively expressing Arabidopsis NPR1 show enhanced resistance to root-knot nematode, Meloidogyne incognita. BMC Research Notes, 4. https://doi.org/10.1186/1756-0500-4-231
Pulavarty, A., Egan, A., Karpinska, A., Horgan, K., & Kakouli-Duarte, T. (2021). Plant parasitic nematodes: a review on their behaviour, host interaction, management approaches and their occurrence in two sites in the republic of Ireland. Plants, 10(11). https://doi.org/10.3390/plants10112352
Putri, A. H., Indarti, S., & Harjaka, T. (2021). Diversity and abundance of nematodes in soil treated with solarization treatments. Biodiversitas Journal of Biological Diversity, 22(7). https://doi.org/10.13057/biodiv/d220708
Ramatsitsi, N., Dube, Z. P., Ramachela, K., & Motloba, T. (2024). Bio-control efficacy of selected indigenous nematophagous fungi against Meloidogyne enterolobii in vitro and on dry bean (Phaseolus vulgaris L.). International Microbiology, 28(1), 151–160. https://doi.org/10.1007/S10123-024-00571-1
Requena, J. L. (2022). Guía técnica: uso de plaguicidas en Panamá: indicación de riesgo e implementación de medidas de mitigación. Ministerio de Desarrollo Agropecuario.
Rosado, F. R., Germano, S., Carbonero, E. R., da Costa, S. M. G., Iacomini, M., & Kemmelmeier, C. (2003). Biomass and exopolysaccharide production in submerged cultures of Pleurotus ostreatoroseus Sing. and Pleurotus ostreatus “florida” (Jack.: Fr.) Kummer. Journal of Basic Microbiology, 43(3), 230–237. https://doi.org/10.1002/jobm.200390026
Rudolph, R. E., Bajek, V., & Munir, M. (2023). Effects of soil solarization and grafting on tomato yield and southern root-knot nematode population densities. HortScience, 58(11), 1443–1449. https://doi.org/10.21273/HORTSCI17396-23
Sahebani, N., & Hadavi, N. (2008). Biological control of the root-knot nematode Meloidogyne javanica by Trichoderma harzianum. Soil Biology and Biochemistry, 40(8), 2016–2020. https://doi.org/10.1016/j.soilbio.2008.03.011
Samara, R. (2022). Evaluation of 11 potential trap crops for root-knot nematode (RKN) control under glasshouse conditions. Open Agriculture, 7(1), 61–68. https://doi.org/10.1515/opag-2022-0074
Sandoval-Ruiz, R., & Grabau, Z. J. (2023). Reniform nematode management using winter crop rotation and residue incorporation methods in greenhouse experiments. Journal of Nematology, 55(1). https://doi.org/10.2478/jofnem-2023-0035
Sato, K., Kadota, Y., & Shirasu, K. (2019). Plant immune responses to parasitic nematodes. Frontiers in Plant Science, 10. https://doi.org/10.3389/fpls.2019.01165
Scholte, K. (2000). Effect of potato used as a trap crop on potato cyst nematodes and other soil pathogens and on the growth of a subsequent main potato crop. Annals of Applied Biology, 136(3), 229–238. https://doi.org/10.1111/j.1744-7348.2000.tb00029.x
Sharon, E., Chet, I., Viterbo, A., Bar-Eyal, M., Nagan, H., Samuels, G. J., & Spiegel, Y. (2007). Parasitism of Trichoderma on Meloidogyne javanica and role of the gelatinous matrix. European Journal of Plant Pathology, 118(3), 247–258. https://doi.org/10.1007/s10658-007-9140-x
Shukuru, B. N., Politaeva, N. A., Sharma, N. R., Akhtar, N., TS, A., & Rana, M. (2024). Bioagents and beyond: harnessing the diversity of nematophagous microorganisms and predators for sustainable management of plant–parasitic nematodes. Journal of Phytopathology, 172(6). https://doi.org/10.1111/jph.70005
Sikandar, A., Zhang, M. Y., Wang, Y. Y., Zhu, X. F., Liu, X. Y., Fan, H. Y., Xuan, Y. H., Chen, L. J., & Duan, Y. X. (2020). Review article: Meloidogyne incognita (root-knot nematode) a risk to agriculture. Applied Ecology and Environmental Research, 18(1). https://doi.org/10.15666/aeer/1801_16791690
Singh, K. P., Stephen, R. A., & Vaish, S. S. (1996). Pathogenicity and development of Catenaria anguillulae on some nematodes. Mycological Research, 100(10), 1204–1206. https://doi.org/10.1016/S0953-7562(96)80181-X
Singh, U. B., Sahu, A., Sahu, N., Singh, R. K., Renu, Prabha, R., Singh, D. P., Sarma, B. K., & Manna, M. C. (2012). Co-inoculation of Dactylaria brochopaga and Monacrosporium eudermatum affects disease dynamics and biochemical responses in tomato (Lycopersicon esculentum Mill.) to enhance bio-protection against Meloidogyne incognita. Crop Protection, 35, 102–109. https://doi.org/10.1016/j.cropro.2012.01.002
Singh, U. B., Sahu, A., Sahu, N., Singh, R. K., Renu, Singh, D. K., Singh, B. P., Jaiswal, R. K., Singh, D. P., Rai, J. P., Manna, M. C., Singh, K. P., Srivastava, J. S., Subba Rao, A., & Rajendra Prasad, S. (2013). Nematophagous fungi: Catenaria anguillulae and Dactylaria brochopaga from seed galls as potential biocontrol agents of Anguina tritici and Meloidogyne graminicola in wheat (Triticum aestivum L.). Biological Control, 67(3), 475–482. https://doi.org/10.1016/j.biocontrol.2013.10.002
Singh, U. B., Sahu, A., Singh, R. K., Singh, D. P., Meena, K. K., Srivastava, J. S., Renu, & Manna, M. C. (2012). Evaluation of biocontrol potential of Arthrobotrys oligospora against Meloidogyne graminicola and Rhizoctonia solani in rice (Oryza sativa L.). Biological Control, 60(3), 262–270. https://doi.org/10.1016/j.biocontrol.2011.10.006
Soares, F. E. de F., Sufiate, B. L., & de Queiroz, J. H. (2018). Nematophagous fungi: far beyond the endoparasite, predator and ovicidal groups. Agriculture and Natural Resources, 52(1). https://doi.org/10.1016/j.anres.2018.05.010
Stapleton, J. J., & DeVay, J. E. (1982). Effect of soil solarization on populations of selected soil-borne microorganisms and growth of deciduous fruit tree seedlings. Phytopathology, 72, 323–326.
Starr, J. L., Bridge, J., & Cook, R. (2002). Resistance to plant-parasitic nematodes: history, current use and future potential. In J. L. Starr, J. Bridge, & R. Cook (Eds.), Plant resistance to parasitic nematodes (pp. 1–22). CABI Publishing. https://doi.org/10.1079/9780851994666.0001
Stirling, G. R. (2011). Biological control of plant-parasitic nematodes: an ecological perspective, a review of progress and opportunities for further research. In K. Davies & Y. Spiegel (Eds.), Control of Plant-Parasitic Nematodes: Progress in Biological Control (Vol. 11, pp. 1–38). Springer Netherlands. https://doi.org/10.1007/978-1-4020-9648-8_1
Stirling, G. R. (2014). Biological control of plant-parasitic nematodes: soil ecosystem management in sustainable agriculture (2nd ed.). CABI. https://doi.org/10.1079/9781780644158.0000
Su, H., Zhao, Y., Zhou, J., Feng, H., Jiang, D., Zhang, K.-Q., & Yang, J. (2017). Trapping devices of nematode-trapping fungi: formation, evolution, and genomic perspectives. Biological Reviews, 92(1), 357–368. https://doi.org/10.1111/brv.12233
Subbotin, S. A., Palomares-Rius, J. E., & Castillo, P. (2021). Taxonomic History. In D. J. Hunt & R. N. Perry (Eds.), Systematics of Root-knot Nematodes (Nematoda: Meloidogynidae) (Vol. 14, pp. 1–3). Brill. https://doi.org/10.1163/9789004387584_002
Subedi, S., Thapa, B., & Shrestha, J. (2020). Root-knot nematode (Meloidogyne incognita) and its management: a review. Journal of Agriculture and Natural Resources, 3(2), 21–31. https://doi.org/10.3126/janr.v3i2.32298
Sun, X., Liao, J., Lu, J., Lin, R., Zou, M., Xie, B., & Cheng, X. (2024). Parasitism of Hirsutella rhossiliensis on different nematodes and its endophytism promoting plant growth and resistance against root-knot nematodes. Journal of Fungi, 10(1), 68. https://doi.org/10.3390/jof10010068
Szabó, M., Csepregi, K., Gálber, M., Virányi, F., & Fekete, C. (2012). Control plant-parasitic nematodes with Trichoderma species and nematode-trapping fungi: The role of chi18-5 and chi18-12 genes in nematode egg-parasitism. Biological Control, 63(2), 121–128. https://doi.org/10.1016/j.biocontrol.2012.06.013
Tazi, H., Hamza, M. A., Hallouti, A., Benjlil, H., Idhmida, A., Furze, J. N., Paulitz, T. C., Mayad, E. H., Boubaker, H., & El Mousadik, A. (2021). Biocontrol potential of nematophagous fungi against Meloidogyne spp. infecting tomato. Organic Agriculture, 11(1). https://doi.org/10.1007/s13165-020-00325-z
Terra, W. C., Campos, V. P., Martins, S. J., Costa, L. S. A. S., da Silva, J. C. P., Barros, A. F., López, L. E., Santos, T. C. N., Smant, G., & Oliveira, D. F. (2018). Volatile organic molecules from Fusarium oxysporum strain 21 with nematicidal activity against Meloidogyne incognita. Crop Protection, 106, 125–131. https://doi.org/10.1016/j.cropro.2017.12.022
Tian, T., Gheysen, G., Kyndt, T., Mo, C., Xiao, X., Lv, Y., Long, H., Wang, G., & Xiao, Y. (2024). Pepper root exudate alleviates cucumber root-knot nematode infection by recruiting a rhizobacterium. Plant Communications, 101139. https://doi.org/10.1016/j.xplc.2024.101139
Townshend, J. L., Meskine, M., & Barron, G. L. (1989). Biological control of Meloidogyne hapla on alfalfa and tomato with the fungus Meria coniospora. Journal of Nematology, 21(2), 179–183.
Trudgill, D. L., & Blok, V. C. (2001). Apomictic, polyphagous root-knot nematodes: exceptionally successful and damaging biotrophic root pathogens. Annu Rev Phytopathol, 39, 53–77. https://doi.org/10.1146/annurev.phyto.39.1.53
Varela-Benavides, I., Durán-Mora, J., & Guzmán-Hernández, T. (2017). Evaluación in vitro de diez cepas de hongos nematófagos para el control de Meloidogyne exigua, Meloidogyne incognita y Radopholus similis. Revista Tecnología En Marcha, 30(1), 27. https://doi.org/10.18845/tm.v30i1.3062
Verdejo, S. (2005). Control biológico de nematodos fitopárasitos. El Control Biológico de Plagas y Enfermedades, 5, 153–166.
Viterbo, A., Inbar, J., Hadar, Y., & Chet, I. (2007). Plant Disease Biocontrol and Induced Resistance via Fungal Mycoparasites. In C. Kubicek & I. Druzhinina (Eds.), The Mycota (pp. 127–146). Springer. https://doi.org/10.1007/978-3-540-71840-6_8
Wan, J., Dai, Z., Zhang, K., Li, G., & Zhao, P. (2021). Pathogenicity and metabolites of endoparasitic nematophagous fungus Drechmeria coniospora YMF1.01759 against nematodes. Microorganisms, 9(8), 1735. https://doi.org/10.3390/microorganisms9081735
Walia, R. K., & Khan, M. R. (2023). Root-knot Nematodes (Meloidogyne spp.). In F. Ahmad & G. N. Blázquez (Eds.), Root-Galling Disease of Vegetable Plants (pp. 1–60). Springer Nature. https://doi.org/10.1007/978-981-99-3892-6_1
Wyss, U., Grundler, F. M. W., & Munch, A. (1992). The parasitic behaviour of second-stage juveniles of Meloidogyne incognita in roots of Arabidopsis thaliana. Nematologica, 38(1–4), 98–111. https://doi.org/10.1163/187529292X00081
Yang, J., Tian, B., Liang, L., & Zhang, K. Q. (2007). Extracellular enzymes and the pathogenesis of nematophagous fungi. Applied Microbiology and Biotechnology, 75(1), 21–31. https://doi.org/10.1007/s00253-007-0881-4
Youssef, M. M. A., & El-Nagdi, W. M. A. (2021). New approach for biocontrolling root-knot nematode, Meloidogyne incognita on cowpea by commercial fresh oyster mushroom (Pleurotus ostreatus). Jordan Journal of Biological Sciences, 14(01), 173–177. https://doi.org/10.54319/jjbs/140122
Zhang, S., Gan, Y., & Xu, B. (2015). Biocontrol potential of a native species of Trichoderma longibrachiatum against Meloidogyne incognita. Applied Soil Ecology, 94, 21–29. https://doi.org/10.1016/j.apsoil.2015.04.010
Zhang, S., Gan, Y., Xu, B., & Xue, Y. (2014). The parasitic and lethal effects of Trichoderma longibrachiatum against Heterodera avenae. Biological Control, 72, 1–8. https://doi.org/10.1016/j.biocontrol.2014.01.009
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Scientia Agropecuaria

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
The authors who publish in this journal accept the following conditions:
a. The authors retain the copyright and assign to the magazine the right of the first publication, with the work registered with the Creative Commons attribution license, which allows third parties to use the published information whenever they mention the authorship of the work and the First publication in this journal.
b. Authors may make other independent and additional contractual arrangements for non-exclusive distribution of the version of the article published in this journal (eg, include it in an institutional repository or publish it in a book) as long as it clearly indicates that the work Was first published in this journal.
c. Authors are encouraged to publish their work on the Internet (for example, on institutional or personal pages) before and during the review and publication process, as it can lead to productive exchanges and a greater and faster dissemination of work Published (see The Effect of Open Access).




