Protocol for in vitro germination and micropropagation of Himatanthus Sucuuba (Spruce ex Müll. arg.) Woodson
DOI:
https://doi.org/10.17268/sci.agropecu.2025.032Keywords:
Tissue culture, growth regulators, in vitro rooting, medicinal species, sucuubaAbstract
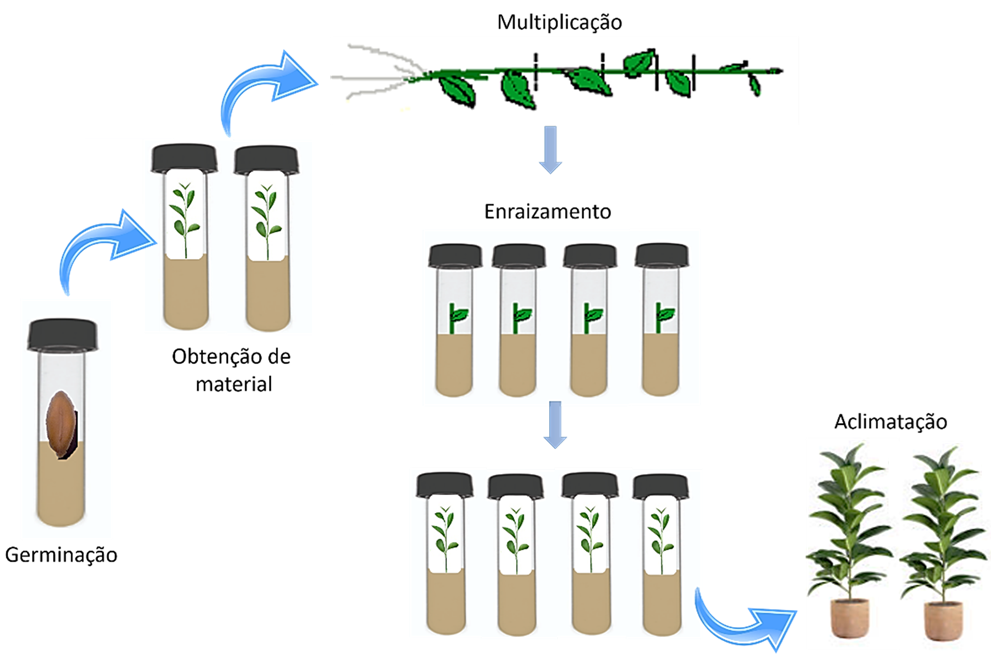
Himatanthus sucuuba is important in folk medicine and is widely used as an antitumor, antifungal, vermifuge and anti-anemic agent1. In this context, the objective of this study was to develop a protocol for in vitro germination and micropropagation of H. sucuuba. The seeds were immersed in a 1.0% (v/v) Cabrio Top solution for one hour on a magnetic stirrer and then in a 0.1% (v/v) diluted NaOCl solution for 30 minutes under agitation, followed by immersion in 70% alcohol for 1 minute. Subsequently, the seeds were rinsed four times with sterile distilled water and then inoculated in MS medium supplemented with the auxins AIA, ANA and AIB at concentrations of 0.0; 1.0; 3.0; 5.0 mg L-1. The experimental design was completely randomized, using 10 treatments with 3 replicates of 10 seeds (n = 30). It was observed that the MS medium supplemented with IAA (5.0 mg L-1) resulted in 80% germination and seedlings with 5.97 cm in height and 4.2 nodal segments. To stimulate rooting, the nodal segments were cut and inoculated in MS medium supplemented with BAP (0.1 mg L-1) and in interaction with the auxins IAA, 2,4-D and ANA, at concentrations of 0.0; 3.0; 5.0 and 8.0 mg L-1 and kept in a growth room at 25 ± 2 °C, with a photoperiod of 16 h. The combination BAP+IAA (0.1 + 8.0 mg L-1) showed the best results with 100% sprouting, 40% callus formation and 30% rooting. In conclusion, in vitro propagation is a promising technique to produce H. sucuuba seedlings, however, hormonal adjustments are necessary.
References
Abd Elaziem, T. M., Ahmed, M. E. salato A. E. naby, & Abou El-Dis, G. R. (2022). In vitro propagation for conservation of the rare date palm (Phoenix dactylifera L.) “Amri” using immature inflorescence. In Vitro Cellular and Developmental Biology - Plant, 58(6), 1048–1056. https://doi.org/10.1007/s11627-022-10296-3
Ai, Y., Chen, Y., Zhu, S., Jiang, L., Chen, J., Li, C., Li, P., Zeng, W., Kuang, D., Liu, Q., & Yang, Y. (2024). The Impacts of Plant Growth Regulators on the Rapid Propagation of Gardenia jasminoides Ellis. in Tissue Culture. Forests, 15(3). https://doi.org/10.3390/f15030446
Alvino, F. de O., & Rayol, B. P. (2007). Efeito de diferentes substratos na germinação de Chroma pyramidale (Cav. ex Lam.) Urb. (Bombacaceae). Ciência Florestal, 17(1), 71–75. https://doi.org/10.5902/198050981937
Boato Da Silva, F. A., Pereira, A. R., Dos, E., & Silveira, S. (2008). Brazilian archives of biology and technology micropropagation of Alibertia edulis Rich. Braz. Arch. Biol. Technol. V, 51(6), 1103–1114.
Braga, F. T., Pasqual, M., Castro, E. M. de, Rafael, G. C., Favero, A. C., & Valente, T. C. T. (2011). Alterações morfofisiolócias de plantas de abacaxizeiro influenciadas por diferentes substratos durante o processo de aclimatização. Ciência e Agrotecnologia, 35(5), 863–868. https://doi.org/10.1590/s1413-70542011000500001
Cordeiro, S. Z., Simas, N. K., Henriques, A. B., & Sato, A. (2014). Micropropagation and callogenesis in Mandevilla guanabarica (Apocynaceae), an endemic plant from Brazil. Crop Breeding and Applied Biotechnology, 14(2), 108–115. https://doi.org/10.1590/1984-70332014v14n2a19
Ferreira, C. da S., Piedade, M. T. F., & Bonates, L. C. (2006). Germinação de sementes e sobrevivência de plântulas de Himatanthus sucuuba (Spruce) Wood. em resposta ao alagamento, nas várzeas da Amazônia Central. Acta Amazonica, 36(4), 413–418. https://doi.org/10.1590/S0044-59672006000400003
Gang, R., Komakech, R., Chung, Y., Okello, D., Kim, W. J., Moon, B. C., Yim, N. H., & Kang, Y. (2023). In vitro propagation of Codonopsis pilosula (Franch.) Nannf. using apical shoot segments and phytochemical assessments of the maternal and regenerated plants. BMC Plant Biology, 23(1), 1–16. https://doi.org/10.1186/s12870-022-03950-w
Herrera-Calderón, O., Calero-Armijos, L. L., Cardona-G, W., Herrera-R, A., Moreno, G., Algarni, M. A., Alqarni, M., & Batiha, G. E. S. (2021). Phytochemical screening of Himatanthus sucuuba (Spruce) woodson (apocynaceae) latex, in vitro cytotoxicity and incision wound repair in mice. Plants, 10(10). https://doi.org/10.3390/plants10102197
Junghans, T. G., & Souza, S. A. (2013). Aspectos prácticos da micropropagação de plantas. 407.
Komakech, R., Kim, Y. G., Kim, W. J., Omujal, F., Yang, S., Moon, B. C., et al. (2020). A micropropagation protocol for the endangered medicinal tree Prunus africana (Hook f.) Kalkman: Genetic fidelity and physiological parameter assessment. Frontiers in Plant Science, 11. https://doi.org/10.3389/fpls.2020.548003
Lemos, S. D. D. C., Santana, I. C., Marques, M., & Albarello, N. (2019). Desenvolvimento e produção in vitro de compostos fenólicos de Ruta graveolens L. exposta a fenantreno e benzo[a]pireno. Revista Virtual de Quimica, 11(5), 1418–1432. https://doi.org/10.21577/1984-6835.20190098
Magalhães, K. do N., Guarniz, W. A. S., Sá, K. M., Freire, A. B., Monteiro, M. P., Nojosa, R. T., Bieski, I. G. C., Custódio, J. B., Balogun, S. O., & Bandeira, M. A. M. (2019). Medicinal plants of the Caatinga, northeastern Brazil: Ethnopharmacopeia (1980–1990) of the late professor Francisco José de Abreu Matos. Journal of Ethnopharmacology, 237(March), 314–353. https://doi.org/10.1016/j.jep.2019.03.032
Merkle, S. A., Koch, J. L., Tull, A. R., Dassow, J. E., Carey, D. W., Barnes, B. F., Richins, M. W. M., et al. (2023). Application of somatic embryogenesis for development of emerald ash borer-resistant white ash and green ash varietals. New Forests, 54(4), 697–720. https://doi.org/10.1007/s11056-022-09903-3
Miranda, N. A., Titon, M., Pereira, I. M., Sebastião, J., Fernandes, C., Santos, M., & Oliveira, R. N. De. (2018). Antioxidants, sucrose and agar in the in vitro multiplication of Eremanthus incanus. Floresta, 48(3), 311–320. https://doi.org/10.5380/rf.v48i3.51365
Murashige, T., & Skoog, F. A. (1962). Revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant, 15, 473–497.
Nilanthi, D., & Yang, Y. (2014). Efeitos da sacarose e outros aditivos no crescimento e desenvolvimento in vitro de coneflower roxo (Echinacea purpurea L.). Av. Biol. 2014, 1–4. https://doi.org/10.1155/2014/402309
Oliveira, L. S. de, Dias, P. C., & Brondani, G. E. (2013). Micropropagação de espécies florestais brasileiras. Pesquisa Florestal Brasileira, 33(76), 439–453. https://doi.org/10.4336/2013.pfb.33.76.481
Oliveira, K. S., Aurélio, F., Freire, D. M., Ahmed, M., & Aloufa, I. (2016a). Efeito de 6-benzilaminopurina e ácido naftalenoacético sobre a propagação in vitro de Hancornia speciosa Gomes. Floresta, 46, 335–342. https://doi.org/10.5380/rf.v46i3.43993
Oliveira, K. S., Freire, F. A. de M., & Aloufa, M. A. I. (2016b). Efeito de 6-benzilaminopurina e ácido naftalenoacético sobre a propagação in vitro de Hancornia speciosa Gomes. Floresta, 46(3), 335–342. https://doi.org/10.5380/rf.v46i3.43993
Patel, D. K. (2023). Biological importance, therapeutic benefits, and analytical aspects of active flavonoidal compounds ‘corylin’ from Psoralea corylifolia in the field of medicine. Infectious Disorders - Drug Targets, 23(1), e250822208005. https://doi.org/10.2174/1871526522666220825160906
Patel, D. K. (2022). Biological importance, therapeutic benefit, and medicinal importance of flavonoid, cirsiliol for the development of remedies against human disorders. Current Bioactive Compounds, 18(3), Article e240821195804. https://doi.org/10.2174/1573407217666210824125427
Santos, T. P., Sá, M. E., Malagutti, E. S., et al. (2022). Effects of gibberellic acid concentration and fruit maturation stage on seed germination and vigor of pitahaya seedlings. Brazilian Journal of Biology. https://doi.org/10.1590/1519-6984.260650
Singh, R., Kharb, P., & Kanta, R. (2011). Rapid micropropagation and callus induction of Catharanthus roseus in vitro using different explants. World Journal of Agricultural Sciences, 7(6), 699–704.
Soares, F. P., Paiva, R., Alvarenga, A. A. de, Nogueira, R. C., Emrich, E. B., & Martinotto, C. (2007). Organogênese direta em explantes caulinares de mangabeira (Hancornia speciosa Gomes). Ciência e Agrotecnologia, 31(4), 1048–1053. https://doi.org/10.1590/s1413-70542007000400016
Souza, K. P. (2017). Propagação in vitro de Himatanthus sucuuba WOOD uma espécie medicinal da amazônia. Universidade do Estado do Amazonas.
Sungkumlong, & Deb, C. R. (2009). Regeneration competence of Tainia latifolia (Lindl.) Benth ex Hook pseudobulb segments: An in vitro study. Indian Journal of Biotechnology, 8(1), 121–126.
Verma, A. K., Singh, R. R., & Singh, S. (2012). Improved alkaloid content in callus culture of Catharanthus roseus. Botanica Serbica, 36 (2), 123–130.
Published
How to Cite
Issue
Section
License
Copyright (c) 2025 Scientia Agropecuaria

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
The authors who publish in this journal accept the following conditions:
a. The authors retain the copyright and assign to the magazine the right of the first publication, with the work registered with the Creative Commons attribution license, which allows third parties to use the published information whenever they mention the authorship of the work and the First publication in this journal.
b. Authors may make other independent and additional contractual arrangements for non-exclusive distribution of the version of the article published in this journal (eg, include it in an institutional repository or publish it in a book) as long as it clearly indicates that the work Was first published in this journal.
c. Authors are encouraged to publish their work on the Internet (for example, on institutional or personal pages) before and during the review and publication process, as it can lead to productive exchanges and a greater and faster dissemination of work Published (see The Effect of Open Access).




