Exploring rhizobial diversity in tara (Caesalpinia spinosa) by trapping with pea (Pisum sativum)
DOI:
https://doi.org/10.17268/sci.agropecu.2024.037Keywords:
Caesalpinia spinosa, Pisum sativum, Rhizobium, 16S-23S rDNA intergenic spacer (IGS), agroforestryAbstract
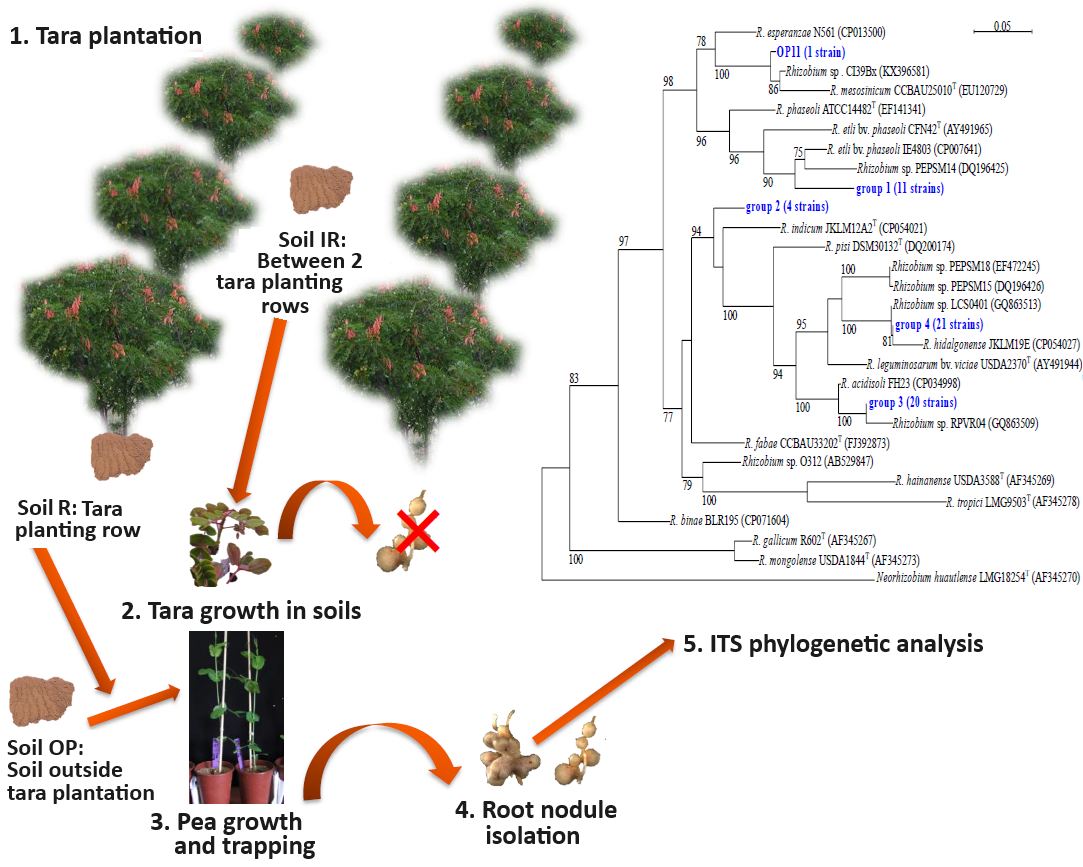
Tara (Caesalpinia spinosa) is an emblematic legume tree of Peruvian dry forests and is a multi-purpose tree for tannins and gum, in particular. Despite its importance, the microbiological aspects associated with tara are not currently considered in forest management, and its nodulation status remains contentious. This study sought to confirm or deny C. spinosa’s nodulation status and, using P. sativum as a trap plant, to investigate the effects of C. spinosa on rhizospheric rhizobial communities. The study revealed a lack of tara nitrogenase activity and that C. spinosa is a non-nodulating species. Soil samples were collected from a tara plantation to investigate their effect on tara and pea growth, in a tara planting row (R), between 2 rows (IR), and outside the plantation (OP). For the total biomass growth parameter, soil R significantly promoted tara and pea growth. For root length and leaf chlorophyll content, there was a significant difference in favor of C. spinosa grown on R and IR soils compared with OP soil. Fifty-seven pea strains were characterized by analyzing the partial 16S-23S rDNA intergenic spacer. The phylogenetic tree showed high diversity with five clusters of Rhizobium spp. in the R. leguminosarum–etli clade and phylogenetic specificity according to soil origin. This study provides information of interest on the non-nodulating nature of C. spinosa and demonstrates the substantial influence of tara on rhizospheric bacterial communities. The results of this study highlight the need to integrate microbiological factors into forest management strategies to improve the ecological sustainability and agricultural yield of tara plantations.
References
Aguilar, O. M., Riva, O., & Peltzer, E. (2004). Analysis of Rhizobium etli and of its symbiosis with wild Phaseolus vulgaris supports coevolution in centers of host diversification. Proceedings of the National Academy of Sciences of the United States of America, 101(37), 13548-13553. https://doi.org/10.1073/pnas.0405805101
Aleman, F. (2009). La tara Caesalpinia spinosa (Mol.) O. Kuntze, especie prodigiosa para los sistemas agroforestales en valles interandinos. Acta Nova, 4(2-3), 300-307.
Ampofo, E. A., Kwakye, P. K., Frimpong, K. A., & Alwa, A. (2016). Irrigation and Bradyrhizobium japonicum Inoculation Effects on Performance of Soybean Production in Tropical Guinea Savanna Zone of Ghana. Journal of Natural Sciences Research, 6, 32.
Balaguer, L., Arroyo-García, R., Jimenez, P., Jimenez, M. D., Villegas, L., et al. (2011). Forest Restoration in a Fog Oasis: Evidence Indicates Need for Cultural Awareness in Constructing the Reference. PLoS ONE, 6(8), e23004. https://doi.org/10.1371/journal.pone.0023004
Belhadi, D., de Lajudie, P., Ramdani, N., Le Roux, C., Boulila, F., et al. (2018). Vicia faba L. in the Bejaia region of Algeria is nodulated by Rhizobium leguminosarum sv. viciae, Rhizobium laguerreae and two new genospecies. Systematic and Applied Microbiology, 41*(2) *, 122-130. https://doi.org/10.1016/j.syapm.2017.10.004
Broughton, W. J., Hernandez, G., Blair, M., et al. (2003). Beans (Phaseolus spp.) – model food legumes. Plant and Soil, 252, 55–128. https://doi.org/10.1023/A:1024146710611
Cordero, I., Jiménez, M. D., Delgado, J. A., Villegas, L., & Balaguer, L. (2016 a). Spatial and demographic structure of tara stands (Caesalpinia spinosa) in Peru: Influence of present and past forest management. Forest Ecology and Management, 377, 71-82. https://doi.org/10.1016/j.foreco.2016.07.032
Cordero, I., Ruiz-Díez, B., Coba de la Pena, T., Balaguer, L., et al. (2016 b). Rhizobial diversity, symbiotic effectiveness and structure of nodules of Vachellia macracantha. Soil Biology and Biochemistry, 96, 39-54. https://doi.org/10.1016/j.soilbio.2016.01.011
Cordeiro, A. B., Ribeiro, R. A., Helene, L. C. F., & Hungria, M. (2017). Rhizobium esperanzae sp. nov., a N2-fixing root symbiont of Phaseolus vulgaris from Mexican soils. International Journal of Systematic and Evolutionary Microbiology, 67 (10), 3937-3945. https://doi.org/10.1099/ijsem.0.002225
Cordero, I., Pueyo, J. J., & Rincón, A. (2024). Bio-fertilisation with native plant growth promoting rhizobacteria increases the tolerance of the neotropical legume tree Caesalpinia spinosa to water deficit. Forest Ecology and Management, 558, 121786. https://doi.org/10.1016/j.foreco.2024.121786
De la Cruz Lapa, P. (2004). Aprovechamiento integral y racional de la tara Caesalpinia spinosa - Caesalpinia tinctoria. An integral and rational utility of tara. Revista del Instituto de Investigación de la Facultad de Minas, Metalurgia y Ciencias Geográficas, 7(14), 64–73. https://doi.org/10.15381/iigeo.v7i14.733
De la Torre, L. (2018). La Tara, beneficios ambientales y recomendaciones para su manejo sostenible en relictos de bosque y sistemas agroforestales. CONDESAN, Quito.
Depret, G., Houot, S., Allard, M.-R., Breuil, M.-C., Nouaim, R., & Laguerre, G. (2004). Long-term effects of crop management on Rhizobium leguminosarum biovar viciae populations. FEMS Microbiology Ecology, 51(1), 87-97.
Dick, R. P. (1994). Soil enzyme activities as indicators of soil quality. In Defining Soil Quality for a Sustainable Environment (pp. 107-124). https://doi.org/10.2136/sssaspecpub35.c7
Dostert, N., Roque, J., Brokamp, G., Cano, A., La Torre, M. I., & Weigend, M. (2009). Drafting botanical monographs (factsheets) for five Peruvian crops. Factsheet: Botanical data of Tara - Caesalpinia spinosa (Molina) Kuntze. https://repositorio.promperu.gob.pe/handle/123456789/1341
Efstathiadou, E., Savvas, D., & Tampakaki, A. P. (2020). Genetic diversity and phylogeny of indigenous rhizobia nodulating faba bean (Vicia faba L.) in Greece. Systematic and Applied Microbiology, 43(6), 126149. https://doi.org/10.1016/j.syapm.2020.126149
El-Idrissi, M. M., Lamin, H., Bouhnik, O., Lamrabet, M., Alami, S., Jabrone, Y., Bennis, M., Bedmar, E. J., & Abdelmoumen, H. (2020). Characterization of Pisum sativum and Vicia faba microsymbionts in Morocco and definition of symbiovar viciae in Rhizobium acidisoli. Systematic and Applied Microbiology, 43, 126084. https://doi.org/10.1016/j.syapm.2020.126084
Flores-Félix, J. D., Carro, L., Cerda-Castillo, E., Squartini, A., Rivas, R., & Velázquez, E. (2020). Analysis of the Interaction between Pisum sativum L. and Rhizobium laguerreae Strains Nodulating This Legume in Northwest Spain. Plants, 9 (12), 1755. https://doi.org/10.3390/plants9121755
Furtak, K., & Gałązka, A. M. (2019). Edaphic Factors and Their Influence on the Microbiological Biodiversity of the Soil Environment. Postępy Mikrobiologii - Advancements of Microbiology, 58, 375-384. https://doi.org/10.21307/pm-2019.58.4.375
García-Fraile, P., Mulas-García, D., Peix, A., Rivas, R., González-Andrés, F., & Velázquez, E. (2010). Phaseolus vulgaris is nodulated in northern Spain by Rhizobium leguminosarum strains harboring two nodC alleles present in American Rhizobium etli strains: Biogeographical and evolutionary implications. Canadian Journal of Microbiology, 56(8), 657-666. https://doi.org/10.1139/w10-048
Gibson, A. H. (1980). Methods for legumes in glasshouse and controlled environment cabinets. In F. J. Bergersen (Ed.), Methods for evaluating biological nitrogen fixation (pp. 139-184). Wiley.
Gouy, M., Tannier, E., Comte, N., & Parsons, D. P. (2021). Seaview Version 5: A Multiplatform Software for Multiple Sequence Alignment, Molecular Phylogenetic Analyses, and Tree Reconciliation. Methods in Molecular Biology, 2231, 241-260. https://doi.org/10.1007/978-1-0716-1036-7_15
Haro, H., Sanon, K. B., Le Roux, C., et al. (2018). Improvement of cowpea productivity by rhizobial and mycorrhizal inoculation in Burkina Faso. Symbiosis, 74, 107–120. https://doi.org/10.1007/s13199-017-0478-3
Hirsch, A. M. (2010). How Rhizobia Survive in the Absence of a Legume Host, a Stressful World Indeed. In J. Seckbach & M. Grube (Eds.), Symbioses and Stress: Joint Ventures in Biology, Cellular Origin, Life in Extreme Habitats and Astrobiology 17, 375-391. https://doi.org/10.1007/978-90-481-9449-018
Ilahi, H., Hsouna, J., Ellouze, W., Gritli, T., Chihaoui, S., et al. (2021). Phylogenetic study of rhizobia nodulating pea (Pisum sativum) isolated from different geographic locations in Tunisia, Systematic and Applied Microbiology, 44(4), 126221. https://doi.org/10.1016/j.syapm.2021.126221
Jaramillo, P. M. D., Guimarães, A. A., Florentino, L. A., et al. (2013). Symbiotic nitrogen-fixing bacterial populations trapped from soils under agroforestry systems in the Western Amazon. Agricultural Microbiology, 70 (6), Dec. https://doi.org/10.1590/S0103-90162013000600004
LPWG, Legume Phylogeny Working Group. (2017). A new subfamily classification of the Leguminosae based on a taxonomically comprehensive phylogeny. Taxon, 66 (1), 44–77. https://doi.org/10.12705/622.8
Mancero, L. (2009). La Tara (Caesalpinia spinosa) en Perú, Bolivia y Ecuador: Análisis de la Cadena Productiva en la Región. Programa Regional ECOBONA-Intercooperation, Quito. https://idus.us.es/xmlui/handle/11441/27400
Marien, J.-N., & Delaunay, G. (2010). La tara, Caesalpinia spinosa: espèce agroforestière emblématique des vallées interandines au Pérou. Bois & Forêts Des Tropiques, 304, 25–33. https://doi.org/10.19182/bft2010.304.a20443
Muniz, A. W., et al. (2017). Symbiotic efficiency of pea (Pisum sativum) rhizobia association under field conditions. African Journal of Agricultural Research, 12(32), 2582-2585. https://doi.org/10.5897/AJAR2017.12257
Nagy, N., Aranyi, N., & Pepo, P. (2017). Investigation of Soybean Production With Microbiological Preparation in the Soil in the West Hungarian Region. Annals of the Academy of Romanian Scientists, Series on Agriculture, Silviculture, and Veterinary Medicine Sciences, 6(1), ISSN 2069-1149.
Ngo Nkot, L., Fankem, H., Adamou, S., Ngakou, A., Nwaga, D., & Etoa, F. X. (2015). Abundance of Legume Nodulating Bacteria in Soils of Diverse Land Use Systems in Cameroon. Universal Journal of Plant Science, 3(5), 97-108.https://doi.org/10.13189/ujps.2015.030502
Ogata Gutiérrez, K. (2006). Diversidad de microorganismos en la rizósfera de "tara" (Caesalpinia spinosa (Molina) Kuntze) y su efecto en el crecimiento del cultivo. [Tesis de título profesional, Universidad Nacional Agraria La Molina, Facultad de Ciencias, Lima].
Ogata, K., & Zúñiga, D. (2008). Estudio de la microflora de la rizosfera de Caesalpinia spinosa en la provincia de Huanuco. Zonas Áridas, 12(1), 191-208.
Ormeño-Orrillo, E., Rogel-Hernández, M. A., Lloret, L., López-López, A., et al. (2012). Change in land use alters the diversity and composition of Bradyrhizobium communities and led to the introduction of Rhizobium etli into the tropical rain forest of Los Tuxtlas (Mexico). Microbial Ecology, 63, 822–834. https://doi.org/10.1007/s00248-011-9974-9
Palmer, K. M., & Young, J. P. W. (2000). Higher diversity of Rhizobium leguminosarum biovar viciae populations in arable soils than in grass soils. Applied and Environmental Microbiology, 66(6), 2445–2450. https://doi.org/10.1128/aem.66.6.2445-2450.2000
Ramírez-Bahena, M. H., García-Fraile, P., Peix, A., et al. (2008). Revision of the taxonomic status of the species Rhizobium leguminosarum (Frank 1879) Frank 1889AL, Rhizobium phaseoli Dangeard 1926 AL and Rhizobium trifolii Dangeard 1926 AL. R. trifolii is a later synonym of R. leguminosarum. Reclassification of the strain R. leguminosarum DSM 30132 (=NCIMB 11478) as Rhizobium pisi sp. nov. International Journal of Systematic and Evolutionary Microbiology, 58(Pt 11), 2484-2490. https://doi.org/10.1099/ijs.0.65621-0
Rahi, P., Giram, P., Chaudhari, D., diCenz, G. C., Kiran, S., et al. (2020). Rhizobium indicum sp. nov., isolated from root nodules of pea (Pisum sativum) cultivated in the Indian trans-Himalayas. Systematic and Applied Microbiology, 43(5), 126127. https://doi.org/10.1016/j.syapm.2020.126127
Rathi, S., Tak, N., Bissa, G., et al. (2018). Selection of Bradyrhizobium or Ensifer symbionts by the native Indian caesalpinioid legume Chamaecrista pumila depends on soil pH and other edaphic and climatic factors. FEMS Microbiology Ecology, 94(11), fiy180. https://doi.org/10.1093/femsec/fiy180
Renier, A., Maillet, F., Fardoux, J., Poinsot, V., Giraud, E., & Nouwen, N. (2011). Photosynthetic Bradyrhizobium Sp. strain ORS285 synthesizes 2-O-methylfucosylated lipochitooligosaccharides for nod gene-dependent interaction with Aeschynomene plants. Molecular Plant-Microbe Interactions, 24(12), 1440-1447. https://doi.org/10.1094/MPMI-05-11-0104
Roman-Ponce, B., Zhang, Y. J., Vasquez-Murrieta, M. S., Sui, X. H., Chen, W. F., Padilla, J. C. A., Guo, X. W., Gao, J. L., Yan, J., Wei Ge, H., & Wang, E. T. (2016). Rhizobium acidisoli sp. nov., isolated from root nodules of Phaseolus vulgaris in acid soils in Mexico. International Journal of Systematic and Evolutionary Microbiology, 66, 398–406. https://doi.org/10.1099/ijsem.0.000732
Sandor, J. A., & Eash, N. S. (1995). Ancient Agricultural Soils in the Andes of Southern Peru. Soil Science Society of America Journal, 59, 170-179. https://doi.org/10.2136/SSSAJ1995.03615995005900010026X
Sharaf, H., Rodrigues, R. R., Moon, J., et al. (2019). Unprecedented bacterial community richness in soybean nodules vary with cultivar and water status. Microbiome, 7, 63. https://doi.org/10.1186/s40168-019-0676-8
Sangay-Tucto, S., & Duponnois, R. (2018). Ecological characteristics of Tara (Caesalpinia spinosa), a multipurpose legume tree of high ecological and commercial value. En P. Gorawala et al. (Eds.), Agricultural Research Updates (Vol. 22). Nova Science Publishers, Inc.
Sangay-Tucto, S., Sanguin, H., Tournier, E., Thioulouse, J., Prin Y., & Duponnois, R. (2017). Impacto de la simbiosis micorrízica arbuscular en el crecimiento temprano de la tara (Caesalpinia spinosa (Molina) Kuntze). Revista Forestal del Perú, 32(2), 89-96. DOI: http://dx.doi.org/10.21704/rfp.v32i2.1040
Santillana, N., Ramirez-Bahena, M. H., García-Fraile, P., Velazquez, E., & Zuniga, D. (2008). Phylogenetic diversity based on rrs, atpD, recA genes and 16S–23S intergenic sequence analyses of rhizobial strains isolated from Vicia faba and Pisum sativum in Peru. Archives of Microbiology, 189, 239–247. https://doi.org/10.1007/s00203-007-0313-y
Simon, Z., Mtei, K., Gessesse, A., & Ndakidemi, P. (2014). Isolation and characterization of nitrogen-fixing rhizobia from cultivated and uncultivated soils of northern Tanzania. American Journal of Plant Sciences, 5, 4050-4067. https://doi.org/10.4236/ajps.2014.526423
Simonsen, A. K., Han, S., Rekret, P., Rentschler, C. S., Heath, K. D., & Stinchcombe, J. R. (2015). Short-term fertilizer application alters phenotypic traits of symbiotic nitrogen-fixing bacteria. Peer J, 3, e1291. https://doi.org/10.7717/peerj.1291
Smykal, P., Kenicer, G., Flavell, A. J., Corander, J., Kosterin, O., et al. (2011). Phylogeny, phylogeography and genetic diversity of the Pisum genus. Plant Genetic Resources, 9(1), 4–18. https://doi.org/10.1017/S147926211000033X
Sprent, J. I., Ardley, J., & James, E. K. (2017). Biogeography of nodulated legumes and their nitrogen-fixing symbionts. New Phytologist, 215, 40–56. https://doi.org/10.1111/nph.14474
Thies, J. E., Woomer, P. L., & Singleton, P. W. (1995). Enrichment of Bradyrhizobium spp. populations in soil due to cropping of the homologous host legume. Soil Biology and Biochemistry, 27, 633-636.
Vincent, J. M. (1970). A manual for the practical study of root-nodule bacteria. En Programme IB (Ed.), International Biological Programme. Blackwell, Oxford: Handbook no. 15. https://trove.nla.gov.au/version/45249216
Wielbo, J., Podlesna, A., Kidaj, D., Podlesny, J., & Skorupska, A. (2015). The Diversity of Pea Microsymbionts in Various Types of Soils and Their Effects on Plant Host Productivity. Microbes Environ, 30(3), 254-261. https://doi.org/10.1264/jsme2.ME14141
Yan, J., Han, X. Z., Ji, Z. J., Li, Y., Wang, E. T., Xie, Z. H., & Chen, W. F. (2014). Abundance and diversity of soybean-nodulating rhizobia in black soil are impacted by land use and crop management. Applied and Environmental Microbiology, 80(17), 5394-5402. https://doi.org/10.1128/AEM.01135-14
Yan, J., Yan, H., Liu, L. X., Chen, W. F., Zhang, X. X., Verastegui-Valdes, M. M., Wang, E. T., & Han, X. Z. (2017). Rhizobium hidalgonense sp. nov., a nodule endophytic bacterium of Phaseolus vulgaris in acid soil. Archives of Microbiology, 199(1), 97-104. https://doi.org/10.1007/s00203-016-1281-x
Yang, C., Yang, J., Li, Y., & Zhou, J. (2008). Genetic diversity of root-nodulating bacteria isolated from pea (Pisum sativum) in subtropical regions of China. Science China Life Sciences, 51(9), 854-862. https://doi.org/10.1007/s11427-008-0104-y
Young, J. P. W., Moeskjær, S., Afonin, A., Rahi, P., et al. (2021). Defining the Rhizobium leguminosarum Species Complex. Genes (Basel), 12(1), 111. https://doi.org/10.3390/genes12010111
Zurita, R., Cadenillas, A., & Gallardo, M. (2021). Influence of Rhizobium and Mycorrhizae in the Production of Seedlings of Caesalpinia Spinosa L. Taya in San Pablo, Peru. ESPOCH Congresses: The Ecuadorian Journal of S.T.E.A.M., 1. https://doi.org/10.18502/espoch.v1i4.9514
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Scientia Agropecuaria

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
The authors who publish in this journal accept the following conditions:
a. The authors retain the copyright and assign to the magazine the right of the first publication, with the work registered with the Creative Commons attribution license, which allows third parties to use the published information whenever they mention the authorship of the work and the First publication in this journal.
b. Authors may make other independent and additional contractual arrangements for non-exclusive distribution of the version of the article published in this journal (eg, include it in an institutional repository or publish it in a book) as long as it clearly indicates that the work Was first published in this journal.
c. Authors are encouraged to publish their work on the Internet (for example, on institutional or personal pages) before and during the review and publication process, as it can lead to productive exchanges and a greater and faster dissemination of work Published (see The Effect of Open Access).




