Polyphenols and theobromine in cacao (Theobroma cacao): Compositional changes across variety, growing region, fermentation, drying and roasting
DOI:
https://doi.org/10.17268/sci.agropecu.2026.002Palabras clave:
cacao chemical composition, polyphenolic compounds, cacao varieties, cacao methylxanthines, postharvest operations, phenolic content, microbial fermentationResumen
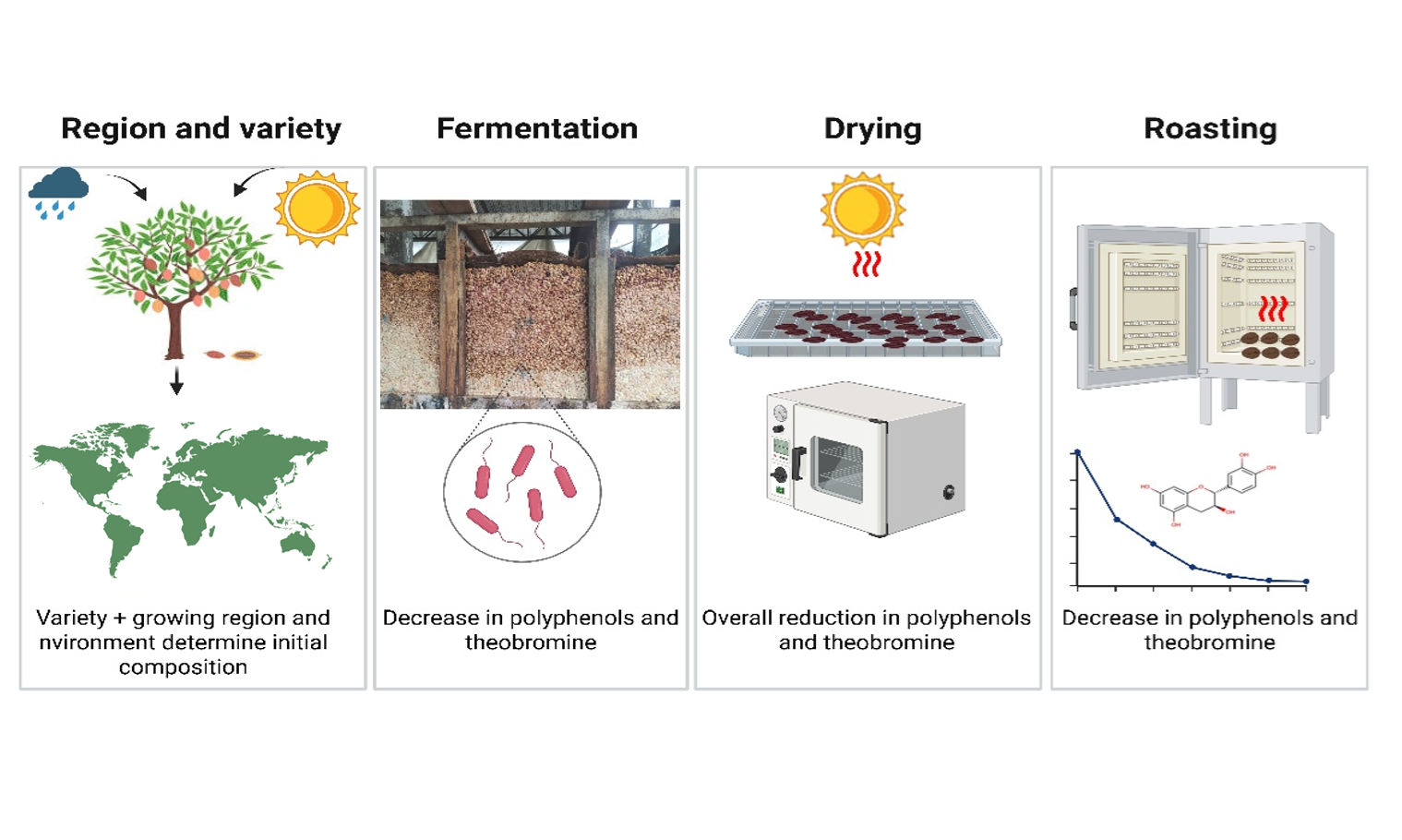
In recent years, cacao and its derivatives have gained significant attention due to their potential health benefits. The primary bioactive compounds in cacao are polyphenols and methylxanthines, predominantly represented by theobromine. Their concentrations vary widely, influenced by cacao variety, growth region, and postharvest processing. Fermentation typically leads to a marked decrease in polyphenols and theobromine, with further reductions during drying and roasting. This review aims to consolidate current knowledge on how these factors affect compound levels, providing insights crucial for optimizing practices to enhance the health benefits and quality of cacao products. Literature consistently shows that cacao properties are shaped by genetics, environmental conditions, and processing stages. Moreover, the unique polyphenol and theobromine profiles can serve as distinctive fingerprints to differentiate cacao origins. Understanding these dynamics is essential for improving both nutritional value and industrial applications.
Citas
Ackar, D., Valek Lendić, K., Valek, M., Šubarić, D., Miličević, B., Babić, J., & Nedić, I. (2013). Cocoa polyphenols: Can we consider cocoa and chocolate as potential functional food? In Journal of Chemistry, Article ID 289392. https://doi.org/10.1155/2013/289392
Agudelo, C., Acevedo, S., Carrillo-Hormaza, L., Galeano, E., & Osorio, E. (2022). Chemometric Classification of Colombian Cacao Crops: Effects of Different Genotypes and Origins in Different Years of Harvest on Levels of Flavonoid and Methylxanthine Metabolites in Raw Cacao Beans. Molecules, 27(7). https://doi.org/10.3390/molecules27072068
Albertini, B., Schoubben, A., Guarnaccia, D., Pinelli, F., Della Vecchia, M., Ricci, M., Di Renzo, G. C., & Blasi, P. (2015). Effect of Fermentation and Drying on Cocoa Polyphenols. Journal of Agricultural and Food Chemistry, 63(45), 9948–9953. https://doi.org/10.1021/acs.jafc.5b01062
Alean, J., Chejne, F., & Rojano, B. (2016). Degradation of polyphenols during the cocoa drying process. Journal of Food Engineering, 189, 99–105. https://doi.org/10.1016/j.jfoodeng.2016.05.026
Aprotosoaie, A. C., Luca, S. V., & Miron, A. (2016). Flavor Chemistry of Cocoa and Cocoa Products-An Overview. Comprehensive Reviews in Food Science and Food Safety, 15(1), 73–91. https://doi.org/10.1111/1541-4337.12180
Ascrizzi, R., Flamini, G., Tessieri, C., & Pistelli, L. (2017). From the raw seed to chocolate: Volatile profile of Blanco de Criollo in different phases of the processing chain. Microchemical Journal, 133, 474–479. https://doi.org/10.1016/j.microc.2017.04.024
Barišić, V., Icyer, N. C., Akyil, S., Toker, O. S., Flanjak, I., & Ačkar, Đ. (2023). Cocoa based beverages – Composition, nutritional value, processing, quality problems and new perspectives. In Trends in Food Science and Technology (Vol. 132, pp. 65–75). Elsevier Ltd. https://doi.org/10.1016/j.tifs.2022.12.011
Bartella, L., Di Donna, L., Napoli, A., Siciliano, C., Sindona, G., & Mazzotti, F. (2019). A rapid method for the assay of methylxanthines alkaloids: Theobromine, theophylline and caffeine, in cocoa products and drugs by paper spray tandem mass spectrometry. Food Chemistry, 278, 261–266. https://doi.org/10.1016/j.foodchem.2018.11.072
Beckett, S. T. (2009). Industrial chocolate manufacture and use. Wiley-Blackwell.
Borja Fajardo, J. G., Horta Tellez, H. B., Peñaloza Atuesta, G. C., Sandoval Aldana, A. P., & Mendez Arteaga, J. J. (2022). Antioxidant activity, total polyphenol content and methylxantine ratio in four materials of Theobroma cacao L. from Tolima, Colombia. Heliyon, 8(5). https://doi.org/10.1016/j.heliyon.2022.e09402
Caligiani, A., Palla, L., Acquotti, D., Marseglia, A., & Palla, G. (2014). Application of 1H NMR for the characterisation of cocoa beans of different geographical origins and fermentation levels. Food Chemistry, 157, 94–99. https://doi.org/10.1016/j.foodchem.2014.01.116
Calvo, A. M., Botina, B. L., García, M. C., Cardona, W. A., Montenegro, A. C., & Criollo, J. (2021). Dynamics of cocoa fermentation and its effect on quality. Scientific Reports, 11(1). https://doi.org/10.1038/s41598-021-95703-2
Camu, N., De Winter, T., Addo, S. K., Takrama, J. S., Bernaert, H., & De Vuyst, L. (2008). Fermentation of cocoa beans: Influence of microbial activities and polyphenol concentrations on the flavour of chocolate. J of the Science of Food and Agriculture, 88(13), 2288–2297. https://doi.org/10.1002/jsfa.3349
Caporaso, N., Whitworth, M. B., Fowler, M. S., & Fisk, I. D. (2018). Hyperspectral imaging for non-destructive prediction of fermentation index, polyphenol content and antioxidant activity in single cocoa beans. Food Chemistry, 258, 343–351. https://doi.org/10.1016/j.foodchem.2018.03.039
Castellanos, J. M., Quintero, C. S., & Carreno, R. (2018). Changes on chemical composition of cocoa beans due to combined convection and infrared radiation on a rotary dryer. IOP Conference Series: Materials Science and Engineering, 437(1). https://doi.org/10.1088/1757-899X/437/1/012011
Chagas Junior, G. C. A., Ferreira, N. R., Gloria, M. B. A., Martins, L. H. da S., & Lopes, A. S. (2021). Chemical implications and time reduction of on-farm cocoa fermentation by Saccharomyces cerevisiae and Pichia kudriavzevii. Food Chemistry, 338. https://doi.org/10.1016/j.foodchem.2020.127834
Colonges, K., Jimenez, J. C., Saltos, A., Seguine, E., Loor Solorzano, R. G., Fouet, O., Argout, X., Assemat, S., Davrieux, F., Cros, E., Boulanger, R., & Lanaud, C. (2021). Two Main Biosynthesis Pathways Involved in the Synthesis of the Floral Aroma of the Nacional Cocoa Variety. Frontiers in Plant Science, 12. https://doi.org/10.3389/fpls.2021.681979
Colonges, K., Seguine, E., Saltos, A., Davrieux, F., Minier, J., et al. (2022). Diversity and determinants of bitterness, astringency, and fat content in cultivated Nacional and native Amazonian cocoa accessions from Ecuador. Plant Genome, 15(4). https://doi.org/10.1002/tpg2.20218
Cortez, D., Flores, M., Calampa, Ll. L., Oliva-Cruz, M., Goñas, M., Meléndez-Mori, J. B., & Chavez, S. G. (2024). From the seed to the cocoa liquor: Traceability of bioactive compounds during the postharvest process of cocoa in Amazonas-Peru. Microchemical Journal, 201. https://doi.org/10.1016/j.microc.2024.110607
Crichton, G. E., Elias, M. F., Dearborn, P., & Robbins, M. (2017). Habitual chocolate intake and type 2 diabetes mellitus in the Maine-Syracuse Longitudinal Study: (1975–2010): Prospective observations. Appetite, 108, 263–269. https://doi.org/10.1016/j.appet.2016.10.008
de Barros Kobi, H., Bragança Alves Fernandes, R., Salgado de Senna, D., Lorrane Rodrigues Borges, L., Cristina Teixeira Ribeiro Vidigal, M., et al. (2024). Metabolic profile of fatty acids, phenolic compounds, and methylxanthines of cocoa kernels (Theobroma cacao L.) from different cultivars produced in cabruca and full sun farming systems. Food Research International, 197. https://doi.org/10.1016/j.foodres.2024.115198
de Mejia, E. G., & Ramirez-Mares, M. V. (2014). Impact of caffeine and coffee on our health. In Trends in Endocrinology and Metabolism (Vol. 25, Issue 10, pp. 489–492). Elsevier Inc. https://doi.org/10.1016/j.tem.2014.07.003
De Taeye, C., Bodart, M., Caullet, G., & Collin, S. (2017). Roasting conditions for preserving cocoa flavan-3-ol monomers and oligomers: interesting behaviour of Criollo clones. Journal of the Science of Food and Agriculture, 97(12), 4001–4008. https://doi.org/10.1002/jsfa.8265
De Taeye, C., Eyamo Evina, V. J., Caullet, G., Niemenak, N., & Collin, S. (2016). Fate of Anthocyanins through Cocoa Fermentation. Emergence of New Polyphenolic Dimers. Journal of Agricultural and Food Chemistry, 64(46), 8876–8885. https://doi.org/10.1021/acs.jafc.6b03892
De Vuyst, L., & Weckx, S. (2016). The cocoa bean fermentation process: from ecosystem analysis to starter culture development. Journal of Applied Microbiology, 121(1), 5–17. https://doi.org/10.1111/jam.13045
Deus, V. L., de Cerqueira E Silva, M. B., Maciel, L. F., Miranda, L. C. R., Hirooka, E. Y., Soares, S. E., de Souza Ferreira, E., & da Silva Bispo, E. (2018). Influence of drying methods on cocoa (Theobroma cacao L.): Antioxidant activity and presence of ochratoxin A. Food Science and Technology (Brazil), 38, 278–285. https://doi.org/10.1590/fst.09917
Di Mattia, C., Martuscelli, M., Sacchetti, G., Scheirlinck, I., Beheydt, B., Mastrocola, D., & Pittia, P. (2013). Effect of Fermentation and Drying on Procyanidins, Antiradical Activity and Reducing Properties of Cocoa Beans. Food and Bioprocess Technology, 6(12), 3420–3432. https://doi.org/10.1007/s11947-012-1028-x
Djikeng, F. T., Teyomnou, W. T., El Tenyang, N., Tiencheu, B., Morfor, A. T., et al. (2018). Effect of traditional and oven roasting on the physicochemical properties of fermented cocoa beans. Heliyon, 4, 533. https://doi.org/10.1016/j.heliyon.2018
do Carmo Brito, B. de N., Campos Chisté, R., da Silva Pena, R., Abreu Gloria, M. B., & Santos Lopes, A. (2017). Bioactive amines and phenolic compounds in cocoa beans are affected by fermentation. Food Chemistry, 228, 484–490. https://doi.org/10.1016/j.foodchem.2017.02.004
Dorta, E., Lobo, M. G., & González, M. (2012). Using drying treatments to stabilise mango peel and seed: Effect on antioxidant activity. LWT, 45(2), 261–268. https://doi.org/10.1016/j.lwt.2011.08.016
D’Souza, R. N., Grimbs, S., Behrends, B., Bernaert, H., Ullrich, M. S., & Kuhnert, N. (2017). Origin-based polyphenolic fingerprinting of Theobroma cacao in unfermented and fermented beans. Food Research International, 99, 550–559. https://doi.org/10.1016/j.foodres.2017.06.007
Dzelagha, B. F., Ngwa, N. M., & Bup, D. N. (2020). A review of cocoa drying technologies and the effect on bean quality parameters. In International Journal of Food Science (Vol. 2020). Hindawi Limited. https://doi.org/10.1155/2020/8830127
Elwers, S., Zambrano, A., Rohsius, C., & Lieberei, R. (2009). Differences between the content of phenolic compounds in Criollo, Forastero and Trinitario cocoa seed (Theobroma cacao L.). European Food Research and Technology, 229(6), 937–948. https://doi.org/10.1007/s00217-009-1132-y
Febrianto, N. A., & Zhu, F. (2019a). Diversity in Composition of Bioactive Compounds among 26 Cocoa Genotypes. Journal of Agricultural and Food Chemistry, 67(34), 9501–9509. https://doi.org/10.1021/acs.jafc.9b03448
Febrianto, N. A., & Zhu, F. (2019b). Intravariety Diversity of Bioactive Compounds in Trinitario Cocoa Beans with Different Degrees of Fermentation. Journal of Agricultural and Food Chemistry, 67(11), 3150–3158. https://doi.org/10.1021/acs.jafc.8b06418
Febrianto, N. A., & Zhu, F. (2020). Changes in the Composition of Methylxanthines, Polyphenols, and Volatiles and Sensory Profiles of Cocoa Beans from the sul 1 Genotype Affected by Fermentation. Journal of Agricultural and Food Chemistry, 68(32), 8658–8675. https://doi.org/10.1021/acs.jafc.0c02909
Fernández-Romero, E., Chavez-Quintana, S. G., Siche, R., Castro-Alayo, E. M., & Cardenas-Toro, F. P. (2020). The kinetics of total phenolic content and monomeric Flavan-3-ols during the roasting process of Criollo Cocoa. Antioxidants, 9(2). https://doi.org/10.3390/antiox9020146
Flores, M. E. J. (2019). Cocoa flavanols: Natural agents with attenuating effects on metabolic syndrome risk factors. Nutrients, 11(4). https://doi.org/10.3390/nu11040751
Giacometti, J., Jolić, S. M., & Josić, D. (2015). Cocoa Processing and Impact on Composition. In Processing and Impact on Active Components in Food (pp. 605–612). Elsevier Inc. https://doi.org/10.1016/B978-0-12-404699-3.00073-1
Gibson, G. R., Hutkins, R., Sanders, M. E., Prescott, S. L., Reimer, R. A., et al. (2017). Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. In Nature Reviews Gastroenterology and Hepatology (Vol. 14, Issue 8, pp. 491–502). Nature Publishing Group. https://doi.org/10.1038/nrgastro.2017.75
Gìltekin-Özgìven, M., Berktaş, I., & Özçelik, B. (2016). Change in stability of procyanidins, antioxidant capacity and in-vitro bioaccessibility during processing of cocoa powder from cocoa beans. LWT, 72, 559–565. https://doi.org/10.1016/j.lwt.2016.04.065
Goya, L., Kongor, J. E., & de Pascual-Teresa, S. (2022). From Cocoa to Chocolate: Effect of Processing on Flavanols and Methylxanthines and Their Mechanisms of Action. In International Journal of Molecular Sciences (Vol. 23, Issue 22). MDPI. https://doi.org/10.3390/ijms232214365
Herman, C., Spreutels, L., Turomzsa, N., Konagano, E. M., & Haut, B. (2018). Convective drying of fermented Amazonian cocoa beans (Theobroma cacao var. Forasteiro). Experiments and mathematical modeling. Food and Bioproducts Processing, 108, 81–94. https://doi.org/10.1016/j.fbp.2018.01.002
Hermund, D. B., Larsen, L. K., Trangbæk, S. R., Madsen, Q. K. R. M. T., Sørensen, A. D. M., Kaya, J., & Jacobsen, C. (2025). Fate of flavonoids and theobromine in cocoa beans during roasting: Effect of time and temperature. JAOCS, Journal of the American Oil Chemists’ Society, 102(1), 35–45. https://doi.org/10.1002/aocs.12853
Hiroshi, A., Misako, K., & Crozier, A. (2011). Methylxanthines (Handbook of Experimental Pharmacology, Volume 200). http://www.springer.com/series/164
Hurst, W. J., Krake, S. H., Bergmeier, S. C., Payne, M. J., Miller, K. B., & Stuart, D. A. (2011). Impact of fermentation, drying, roasting and Dutch processing on flavan-3-ol stereochemistry in cacao beans and cocoa ingredients. Chemistry Central Journal, 5(1). https://doi.org/10.1186/1752-153X-5-53
Ioannone, F., Di Mattia, C. D., De Gregorio, M., Sergi, M., Serafini, M., & Sacchetti, G. (2015). Flavanols, proanthocyanidins and antioxidant activity changes during cocoa (Theobroma cacao L.) roasting as affected by temperature and time of processing. Food Chemistry, 174, 256–262. https://doi.org/10.1016/j.foodchem.2014.11.019
Jaćimović, S., Popović-Djordjević, J., Sarić, B., Krstić, A., Mickovski-Stefanović, V., & Pantelić, N. (2022). Antioxidant Activity and Multi-Elemental Analysis of Dark Chocolate. Foods, 11(10). https://doi.org/10.3390/foods11101445
Jalil, A. M. M., & Ismail, A. (2008). Polyphenols in Cocoa and Cocoa Products: Is There a Link between Antioxidant Properties and Health? Molecules 2008, Vol. 13, Pages 2190-2219, 13(9), 2190–2219. https://doi.org/10.3390/MOLECULES13092190
Jean-Marie, E., Bereau, D., & Robinson, J. C. (2021). Benefits of polyphenols and methylxanthines from cocoa beans on dietary metabolic disorders. In Foods (Vol. 10, Issue 9). MDPI. https://doi.org/10.3390/foods10092049
Jinap, S., Nazamid, S., & Jamilah, B. (2002). Activation of remaining key enzymes in dried under-fermented cocoa beans and its effect on aroma precursor formation. Food Chemistry, 78, 407–417. https://doi.org/https://doi.org/10.1016/S0308-8146(02)00120-6
Joel, N., Pius, B., Deborah, A., & Chris, U. (2013). Production and quality evaluation of cocoa products (plain cocoa powder and chocolate). American Journal of Food and Nutrition , 31–38. https://doi.org/10.5251/ajfn.2013.3.1.31.38
Kongor, J. E., Hinneh, M., de Walle, D. Van, Afoakwa, E. O., Boeckx, P., & Dewettinck, K. (2016). Factors influencing quality variation in cocoa (Theobroma cacao) bean flavour profile - A review. In Food Research International (Vol. 82, pp. 44–52). Elsevier Ltd. https://doi.org/10.1016/j.foodres.2016.01.012
Kouam, J. C. D., Ndjaga, J. M., Akoa, S. P., Ondobo, M. L., Onomo, P. E., Djocgoue, P. F., Niemenak, N., & Collin, S. (2022). Flavan-3-ol and flavonol analysis in healthy and infected parents and progenies of cocoa leaves (Theobroma cacao L.) with Phytophthora megakarya Bras. and Grif. Tropical Plant Pathology, 47(5), 646–658. https://doi.org/10.1007/s40858-022-00521-0
Krähmer, A., Engel, A., Kadow, D., Ali, N., Umaharan, P., Kroh, L. W., & Schulz, H. (2015). Fast and neat - Determination of biochemical quality parameters in cocoa using near infrared spectroscopy. Food Chemistry, 181, 152–159. https://doi.org/10.1016/j.foodchem.2015.02.084
Lachenaud, P., & Motamayor, J. C. (2017). The Criollo cacao tree (Theobroma cacao L.): a review. Genetic Resources and Crop Evolution, 64(8), 1807–1820. https://doi.org/10.1007/s10722-017-0563-8
Lavorgna, M., Pacifico, S., Nugnes, R., Russo, C., Orlo, E., Piccolella, S., & Isidori, M. (2021). Theobroma cacao criollo var. Beans: Biological properties and chemical profile. Foods, 10(3). https://doi.org/10.3390/foods10030571
Lemarcq, V., Tuenter, E., Bondarenko, A., Van de Walle, D., De Vuyst, L., Pieters, L., Sioriki, E., & Dewettinck, K. (2020). Roasting-induced changes in cocoa beans with respect to the mood pyramid. Food Chemistry, 332. https://doi.org/10.1016/j.foodchem.2020.127467
Li, Y., Feng, Y., Zhu, S., Luo, C., Ma, J., & Zhong, F. (2012). The effect of alkalization on the bioactive and flavor related components in commercial cocoa powder. Journal of Food Composition and Analysis, 25(1), 17–23. https://doi.org/10.1016/j.jfca.2011.04.010
Lima, Lí. J. R., Almeida, M. H., Rob Nout, M. J., & Zwietering, M. H. (2011). Theobroma cacao L., “the food of the gods”: Quality determinants of commercial cocoa beans, with particular reference to the impact of fermentation. Critical Reviews in Food Science and Nutrition, 51(8), 731–761. https://doi.org/10.1080/10408391003799913
Loureiro, G. A. H. A., Araujo, Q. R., Sodré, G. A., Valle, R. R., Souza, J. O., Ramos, E. M. L. S., Comerford, N. B., & Grierson, P. F. (2017). Cacao quality: Highlighting selected attributes. In Food Reviews International (Vol. 33, Issue 4, pp. 382–405). Taylor and Francis Inc. https://doi.org/10.1080/87559129.2016.1175011
Maldonado, Y. E., & Figueroa, J. G. (2023). Microwave-Assisted Extraction Optimization and Effect of Drying Temperature on Catechins, Procyanidins and Theobromine in Cocoa Beans. Molecules, 28(9). https://doi.org/10.3390/molecules28093755
McClure, A. P., Spinka, C. M., & Grün, I. U. (2021). Quantitative analysis and response surface modeling of important bitter compounds in chocolate made from cocoa beans with eight roast profiles across three origins. Journal of Food Science, 86(11), 4901–4913. https://doi.org/10.1111/1750-3841.15924
Mihai, R. A., Landazuri Abarca, P. A., Tinizaray Romero, B. A., Florescu, L. I., Catană, R., & Kosakyan, A. (2022). Abiotic Factors from Different Ecuadorian Regions and Their Contribution to Antioxidant, Metabolomic and Organoleptic Quality of Theobroma cacao L. Beans, Variety “Arriba Nacional.” Plants, 11(7). https://doi.org/10.3390/plants11070976
Nazaruddin, R., Seng, L. K., Hassan, O., & Said, M. (2006). Effect of pulp preconditioning on the content of polyphenols in cocoa beans (Theobroma Cacao) during fermentation. Industrial Crops and Products, 24(1), 87–94. https://doi.org/10.1016/j.indcrop.2006.03.013
Nehlig, A. (2013). The neuroprotective effects of cocoa flavanol and its influence on cognitive performance. British Journal of Clinical Pharmacology, 75(3), 716–727. https://doi.org/10.1111/j.1365-2125.2012.04378.x
Nieto-Figueroa, K. H., Mendoza-García, N. V., Gaytán-Martínez, M., Wall-Medrano, A., Guadalupe Flavia Loarca-Piña, M., & Campos-Vega, R. (2020). Effect of drying methods on the gastrointestinal fate and bioactivity of phytochemicals from cocoa pod husk: In vitro and in silico approaches. Food Research International, 137. https://doi.org/10.1016/j.foodres.2020.109725
Oracz, J., & Nebesny, E. (2016). Antioxidant Properties of Cocoa Beans (Theobroma cacao L.): Influence of Cultivar and Roasting Conditions. International Journal of Food Properties, 19(6), 1242–1258. https://doi.org/10.1080/10942912.2015.1071840
Oracz, J., Nebesny, E., & Żyżelewicz, D. (2015a). Changes in the flavan-3-ols, anthocyanins, and flavanols composition of cocoa beans of different Theobroma cacao L. groups affected by roasting conditions. European Food Research and Technology, 241(5), 663–681. https://doi.org/10.1007/s00217-015-2494-y
Oracz, J., Zyzelewicz, D., & Nebesny, E. (2015b). The Content of Polyphenolic Compounds in Cocoa Beans (Theobroma cacao L.), Depending on Variety, Growing Region, and Processing Operations: A Review. Critical Reviews in Food Science and Nutrition, 55(9), 1176–1192. https://doi.org/10.1080/10408398.2012.686934
Osorio-Guarín, J. A., Berdugo-Cely, J., Coronado, R. A., Zapata, Y. P., Quintero, C., Gallego-Sánchez, G., & Yockteng, R. (2017). Colombia a source of cacao genetic diversity as revealed by the population structure analysis of germplasm bank of theobroma cacao l. Frontiers in Plant Science, 8. https://doi.org/10.3389/fpls.2017.01994
Pagliari, S., Celano, R., Rastrelli, L., Sacco, E., Arlati, F., Labra, M., & Campone, L. (2022). Extraction of methylxanthines by pressurized hot water extraction from cocoa shell by-product as natural source of functional ingredient. LWT, 170. https://doi.org/10.1016/j.lwt.2022.114115
Pedan, V., Fischer, N., & Rohn, S. (2016). An online NP-HPLC-DPPH method for the determination of the antioxidant activity of condensed polyphenols in cocoa. Food Research International, 89, 890–900. https://doi.org/10.1016/j.foodres.2015.10.030
Perez, M., Lopez-Yerena, A., & Vallverdú-Queralt, A. (2021). Traceability, authenticity and sustainability of cocoa and chocolate products: a challenge for the chocolate industry. In Critical Reviews in Food Science and Nutrition (Vol. 62, Issue 2, pp. 475–489). Taylor and Francis Ltd. https://doi.org/10.1080/10408398.2020.1819769
Rabadan-Chávez, G., Quevedo-Corona, L., Garcia, A. M., Reyes-Maldonado, E., & Jaramillo-Flores, M. E. (2016). Cocoa powder, cocoa extract and epicatechin attenuate hypercaloric diet-induced obesity through enhanced β-oxidation and energy expenditure in white adipose tissue. Journal of Functional Foods, 20, 54–67. https://doi.org/10.1016/j.jff.2015.10.016
Racine, K. C., Lee, A. H., Wiersema, B. D., Huang, H., Lambert, J. D., Stewart, A. C., & Neilson, A. P. (2019). Development and characterization of a pilot-scale model cocoa fermentation system suitable for studying the impact of fermentation on putative bioactive compounds and bioactivity of cocoa. Foods, 8(3). https://doi.org/10.3390/foods8030102
Ramos-Escudero, F., Casimiro-Gonzales, S., Cádiz-Gurrea, M. de la L., Cancino Chávez, K., Basilio-Atencio, J., et al. (2023). Optimizing vacuum drying process of polyphenols, flavanols and DPPH radical scavenging assay in pod husk and bean shell cocoa. Scientific Reports, 13(1). https://doi.org/10.1038/s41598-023-40815-0
Rawel, H. M., Huschek, G., Sagu, S. T., & Homann, T. (2019). Cocoa Bean Proteins—Characterization, Changes and Modifications due to Ripening and Post-Harvest Processing. Nutrients 2019, Vol. 11, Page 428, 11(2), 428. https://doi.org/10.3390/NU11020428
Rojas, L. F., Gallego, A., Gil, A., Londoño, J., & Atehortúa, L. (2015). Monitoring accumulation of bioactive compounds in seeds and cell culture of Theobroma cacao at different stages of development. In Vitro Cellular and Developmental Biology - Plant, 51(2), 174–184. https://doi.org/10.1007/s11627-015-9684-y
Rojas, M., Hommes, A., Heeres, H. J., & Chejne, F. (2022). Physicochemical Phenomena in the Roasting of Cocoa (Theobroma cacao L.). In Food Engineering Reviews (Vol. 14, Issue 3, pp. 509–533). Springer. https://doi.org/10.1007/s12393-021-09301-z
Romanens, E., Näf, R., Lobmaier, T., Pedan, V., Leischtfeld, S. F., Meile, L., & Schwenninger, S. M. (2018). A lab-scale model system for cocoa bean fermentation. Applied Microbiology and Biotechnology, 102(7), 3349–3362. https://doi.org/10.1007/s00253-018-8835-6
Romero-Cortes, T., Salgado-Cervantes, M. A., García-Alamilla, P., García-Alvarado, M. A., del C Rodríguez-Jimenes, G., Hidalgo-Morales, M., & Robles-Olvera, V. (2013). Relationship between fermentation index and other biochemical changes evaluated during the fermentation of Mexican cocoa (Theobroma cacao) beans. Journal of the Science of Food and Agriculture, 93(10), 2596–2604. https://doi.org/10.1002/jsfa.6088
Samaniego, I., Espín, S., Quiroz, J., Ortiz, B., Carrillo, W., García-Viguera, C., & Mena, P. (2020). Effect of the growing area on the methylxanthines and flavan-3-ols content in cocoa beans from Ecuador. Journal of Food Composition and Analysis, 88(November 2019), 103448. https://doi.org/10.1016/j.jfca.2020.103448
Sanchez-Capa, M., Viteri-Sanchez, S., Burbano-Cachiguango, A., Abril-Donoso, M., Vargas-Tierras, T., Suarez-Cedillo, S., & Mestanza-Ramón, C. (2022). New Characteristics in the Fermentation Process of Cocoa (Theobroma cacao L.) “Super Árbol” in La Joya de los Sachas, Ecuador. Sustainability (Switzerland), 14(13). https://doi.org/10.3390/su14137564
Sandhya, M. V. S., Yallappa, B. S., Varadaraj, M. C., Puranaik, J., Rao, L. J., Janardhan, P., & Murthy, P. S. (2016). Inoculum of the starter consortia and interactive metabolic process in enhancing quality of cocoa bean (Theobroma cacao) fermentation. LWT, 65, 731–738. https://doi.org/10.1016/j.lwt.2015.09.002
Santander Muñoz, M., Rodríguez Cortina, J., Vaillant, F. E., & Escobar Parra, S. (2020). An overview of the physical and biochemical transformation of cocoa seeds to beans and to chocolate: Flavor formation. In Critical Reviews in Food Science and Nutrition (Vol. 60, Issue 10, pp. 1593–1613). Taylor and Francis Inc. https://doi.org/10.1080/10408398.2019.1581726
Scapagnini, G., Davinelli, S., Drago, F., De Lorenzo, A., & Oriani, G. (2012). Antioxidants as antidepressants: Fact or fiction? In CNS Drugs (Vol. 26, Issue 6, pp. 477–490). https://doi.org/10.2165/11633190-000000000-00000
Schlüter, A., André, A., Hühn, T., Rohn, S., & Chetschik, I. (2022). Influence of Aerobic and Anaerobic Moist Incubation on Selected Nonvolatile Constituents-Comparison to Traditionally Fermented Cocoa Beans. Journal of Agricultural and Food Chemistry, 70(51), 16335–16346. https://doi.org/10.1021/acs.jafc.2c06493
Schwan, R. F., Pereira, G. V. de M., & Fleet, G. H. (2014). Microbial activities during cocoa fermentation. Cocoa and Coffee Fermentations, January 2014, 129–192.
Soares, T. F., & Oliveira, M. B. P. P. (2022). Cocoa By-Products: Characterization of Bioactive Compounds and Beneficial Health Effects. Molecules (Basel, Switzerland), 27(5). https://doi.org/10.3390/MOLECULES27051625
Spizzirri, U. G., Ieri, F., Campo, M., Paolino, D., Restuccia, D., & Romani, A. (2019). Biogenic amines, phenolic, and aroma-related compounds of unroasted and roasted cocoa beans with different origin. Foods, 8(8). https://doi.org/10.3390/foods8080306
Stanley, T. H., Van Buiten, C. B., Baker, S. A., Elias, R. J., Anantheswaran, R. C., & Lambert, J. D. (2018). Impact of roasting on the flavan-3-ol composition, sensory-related chemistry, and in vitro pancreatic lipase inhibitory activity of cocoa beans. Food Chemistry, 255, 414–420. https://doi.org/10.1016/j.foodchem.2018.02.036
Suazo, Y., Davidov-Pardo, G., & Arozarena, I. (2014). Effect of Fermentation and Roasting on the Phenolic Concentration and Antioxidant Activity of Cocoa from Nicaragua. Journal of Food Quality, 37(1), 50–56. https://doi.org/10.1111/jfq.12070
Teh, Q. T. M., Tan, G. L. Y., Loo, S. M., Azhar, F. Z., Menon, A. S., & Hii, C. L. (2016). The Drying Kinetics and Polyphenol Degradation of Cocoa Beans. Journal of Food Process Engineering, 39(5), 484–491. https://doi.org/10.1111/jfpe.12239
Todorovic, V., Milenkovic, M., Vidovic, B., Todorovic, Z., & Sobajic, S. (2017). Correlation between Antimicrobial, Antioxidant Activity, and Polyphenols of Alkalized/Nonalkalized Cocoa Powders. Journal of Food Science, 82(4), 1020–1027. https://doi.org/10.1111/1750-3841.13672
Tušek, K., Valinger, D., Jurina, T., Sokač Cvetnić, T., Gajdoš Kljusurić, J., & Benković, M. (2024). Bioactives in Cocoa: Novel Findings, Health Benefits, and Extraction Techniques. In Separations (Vol. 11, Issue 4). Multidisciplinary Digital Publishing Institute (MDPI). https://doi.org/10.3390/separations11040128
Utrilla-Vázquez, M., Rodríguez-Campos, J., Avendaño-Arazate, C. H., Gschaedler, A., & Lugo-Cervantes, E. (2020). Analysis of volatile compounds of five varieties of Maya cocoa during fermentation and drying processes by Venn diagram and PCA. Food Research International, 129. https://doi.org/10.1016/j.foodres.2019.108834
Wang, M., Wei, Y., Ji, B., & Nielsen, J. (2020). Advances in Metabolic Engineering of Saccharomyces cerevisiae for Cocoa Butter Equivalent Production. In Frontiers in Bioengineering and Biotechnology (Vol. 8). Frontiers Media S.A. https://doi.org/10.3389/fbioe.2020.594081
Williamson, G. (2017). The role of polyphenols in modern nutrition. In Nutrition Bulletin (Vol. 42, Issue 3, pp. 226–235). Blackwell Publishing Ltd. https://doi.org/10.1111/nbu.12278
Zapata Bustamante, S., Tamayo Tenorio, A., & Alberto Rojano, B. (2015). Efecto del Tostado Sobre los Metabolitos Secundarios y la Actividad Antioxidante de Clones de Cacao Colombiano. Revista Facultad Nacional de Agronomía Medellín, 68(1), 7497–7507. https://doi.org/10.15446/rfnam.v68n1.47836
Zhang, M., Zhang, H., Jia, L., Zhang, Y., Qin, R., Xu, S., & Mei, Y. (2024). Health benefits and mechanisms of theobromine. In Journal of Functional Foods (Vol. 115). Elsevier Ltd. https://doi.org/10.1016/j.jff.2024.106126
Zheng, X.-Q., Koyama, Y., Nagai, C., & Ashihara, H. (2004). Biosynthesis, accumulation and degradation of theobromine in developing Theobroma cacao fruits. J. Plant Physiol, 161, 363–369. https://doi.org/https://doi.org/10.1078/0176-1617-01253
Żyżelewicz, D., Budryn, G., Oracz, J., Antolak, H., Kręgiel, D., & Kaczmarska, M. (2018). The effect on bioactive components and characteristics of chocolate by functionalization with raw cocoa beans. Food Research International, 113, 234–244. https://doi.org/10.1016/j.foodres.2018.07.017
Zzaman, W., Bhat, R., & Yang, T. A. (2014). Effect of superheated steam roasting on the phenolic antioxidant properties of cocoa beans. Journal of Food Processing and Preservation, 38(4), 1932–1938. https://doi.org/10.1111/jfpp.12166
Descargas
Publicado
Cómo citar
Número
Sección
Licencia
Derechos de autor 2025 Scientia Agropecuaria

Esta obra está bajo una licencia internacional Creative Commons Atribución-NoComercial 4.0.
Los autores que publican en esta revista aceptan los siguientes términos:
a. Los autores conservan los derechos de autor y conceden a la revista el derecho publicación, simultáneamente licenciada bajo una licencia de Creative Commons que permite a otros compartir el trabajo, pero citando la publicación inicial en esta revista.
b. Los autores pueden celebrar acuerdos contractuales adicionales separados para la distribución no exclusiva de la versión publicada de la obra de la revista (por ejemplo, publicarla en un repositorio institucional o publicarla en un libro), pero citando la publicación inicial en esta revista.
c. Se permite y anima a los autores a publicar su trabajo en línea (por ejemplo, en repositorios institucionales o en su sitio web) antes y durante el proceso de presentación, ya que puede conducir a intercambios productivos, así como una mayor citación del trabajo publicado (ver efecto del acceso abierto).




