Antimicrobianos en la salmonicultura global: Resistencia, efecto en la salud humana y medio ambiente, y perspectivas futuras
DOI:
https://doi.org/10.17268/sci.agropecu.2024.007Palabras clave:
antimicrobianos, cultivo salmón, mecanismos de resistencia, microbiota, sedimento marinoResumen
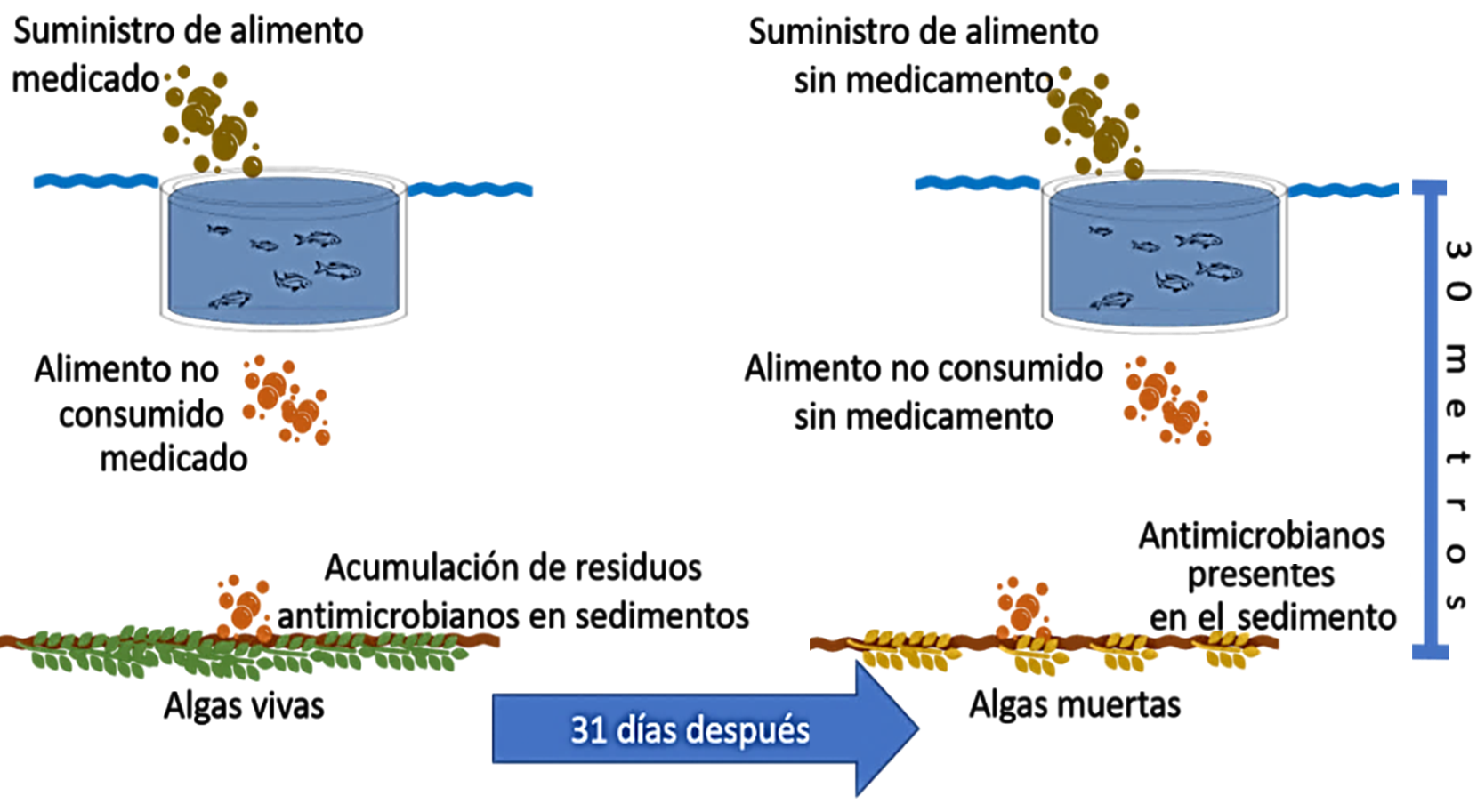
La salmonicultura frente a la resistencia de los antimicrobianos y los efectos sobre la salud humana y medio ambiente. Resumen: La presente revisión recopila y analiza estudios de los últimos 5 años sobre uso de antimicrobianos en la industria del salmón, efectos reportados en peces y seres humanos, y los impactos sobre el medio ambiente. Existe una creciente preocupación respecto al manejo actual de los planes terapéuticos veterinarios empleados para el tratamiento y control de infecciones bacterianas. Las principales problemáticas están relacionadas con el aumento en la resistencia hacia antimicrobianos por parte comunidades bacterianas patógenas y no patógenas, la transferencia horizontal de genes de resistencia entre células bacterianas y, un mayor número de casos de tratamientos médicos fallidos en poblaciones humanas y animales. Por lo tanto, los desafíos a futuro serán reducir la dependencia hacia los antimicrobianos en producciones animales mediante la investigación de tecnologías alternativas, la recopilación de información epidemiológica de sectores productivos que permitan realizar monitoreo detallado en las diferentes fases de cultivo, medidas de bioseguridad desde un enfoque de “One Health” y, una supervisión estricta en la formulación de medicamentos por parte de los profesionales en el área animal.
Citas
Avendaño‐Herrera, R., Mancilla, M., & Miranda, C. D. (2023). Use of antimicrobials in Chilean Salmon farming: Facts, myths and perspectives. Reviews in Aquaculture, 15(1), 89-111. https://doi.org/10.1111/raq.12702
Azanu, D., Styrishave, B., Darko, G., Weisser, J. J., & Abaidoo, R. C. (2018). Occurrence and risk assessment of antibiotics in water and lettuce in Ghana. Science of the Total Environment, 622, 293-305. https://doi.org/10.1016/j.scitotenv.2017.11.287
Bacanli, M., & Başaran, N. (2019). Importance of antibiotic residues in animal food. Food and Chemical Toxicology, 125, 462–466. https://doi.org/10.1016/j.fct.2019.01.033
BCGlobal (2022). Veterinary Drugs. https://www.bryantchristie.com/BCGlobal-Subscriptions/Veterinary-Drugs
Blair, S. D., & Glover, C. N. (2019). Acute exposure of larval rainbow trout (Oncorhynchus mykiss) to elevated temperature limits hsp70b expression and influences future thermotolerance. Hydrobiologia, 836, 155-167. https://doi.org/10.1007/s10750-019-3948-1
Bruce, T. J., Ma, J., Knupp, C., Loch, T. P., Faisal, M., & Cain, K. D. (2020). Cross‐protection of a live‐attenuated Flavobacterium psychrophilum immersion vaccine against novel Flavobacterium spp. and Chryseobacterium spp. strains. Journal of fish diseases, 43(8), 915-928. https://doi.org/10.1111/jfd.13201
Burridge, L., Weis, J. S., Cabello, F., Pizarro, J., & Bostick, K. (2010). Chemical use in salmon aquaculture: a review of current practices and possible environmental effects. Aquaculture, 306(1-4), 7-23. https://doi.org/10.1016/j.aquaculture.2010.05.020
Buschmann, A. H., Tomova, A., López, A., Maldonado, M. A., Henríquez, L. A., Ivanova, L., Moy, F., Godfrey, H. P., & Cabello, F. C. (2012). Salmon aquaculture and antimicrobial resistance in the marine environment. PLoS ONE, 7(8), 26–28. https://doi.org/10.1371/journal.pone.0042724
Cabello, F. C., & Godfrey, H. P. (2023). One Health: Piscirickettsia salmonis, salmon overproduction and antimicrobial use and resistance. Aquaculture, 739800. https://doi.org/10.1016/j.aquaculture.2023.739800
Cabello, F. C., & Godfrey, H. P. (2019). Salmon aquaculture, Piscirickettsia salmonis virulence, and One Health: Dealing with harmful synergies between heavy antimicrobial use and piscine and human health. Aquaculture, 507, 451-456. https://doi.org/10.1016/j.aquaculture.2019.04.048
Cabello, F. C., Godfrey, H. P., Ivanova, L., Shah, S. Q. A., Sørum, H., & Tomova, A. (2020). Freshwater salmon aquaculture in Chile and transferable antimicrobial resistance. Environmental Microbiology, 22(2), 559–563. https://doi.org/10.1111/1462-2920.14891
Cabello, F. C., Godfrey, H. P., Tomova, A., Ivanova, L., Dölz, H., Millanao, A., & Buschmann, A. H. (2013). Antimicrobial use in aquaculture re-examined: Its relevance to antimicrobial resistance and to animal and human health. Environmental Microbiology, 15(7), 1917–1942. https://doi.org/10.1111/1462-2920.12134
Cabello, F. C., Millanao, A. R., Lozano‐Muñoz, I., & Godfrey, H. P. (2023). Misunderstandings and misinterpretations: Antimicrobial use and resistance in salmon aquaculture. Environmental Microbiology Reports. https://doi.org/10.1111/1758-2229.13147
Cabello, F. C. (2006). Heavy use of prophylactic antibiotics in aquaculture: a growing problem for human and animal health and for the environment. Environmental microbiology, 8(7), 1137-1144. https://doi.org/10.1111/j.1462-2920.2006.01054.x
Capone, D. G., Weston, D. P., Miller, V., & Shoemaker, C. (1996). Antibacterial residues in marine sediments and invertebrates following chemotherapy in aquaculture. Aquaculture, 145(1-4), 55-75. https://doi.org/10.1016/S0044-8486(96)01330-0
Caputo, A., Bondad‐Reantaso, M. G., Karunasagar, I., Hao, B., Gaunt, P., et al. (2022). Antimicrobial resistance in aquaculture: A global analysis of literature and national action plans. Reviews in Aquaculture. https://doi.org/10.1111/raq.12741
Chen, J., Ying, G. G., & Deng, W. J. 2019. Antibiotic Residues in Food: Extraction, Analysis, and Human Health Concerns. Journal of Agricultural and Food Chemistry, 67(27), 7569–7586. https://doi.org/10.1021/acs.jafc.9b01334
Chen, K., & Zhou, J. L. (2014). Occurrence and behavior of antibiotics in water and sediments from the Huangpu River, Shanghai, China. Chemosphere, 95, 604-612. https://doi.org/10.1016/j.chemosphere.2013.09.119
Chi, T. T. K., Clausen, J. H., Van, P. T., Tersbøl, B., & Dalsgaard, A. (2017). Use practices of antimicrobials and other compounds by shrimp and fish farmers in Northern Vietnam. Aquaculture Reports, 7, 40-47. https://doi.org/10.1016/j.aqrep.2017.05.003
Chiesa, L. M., Nobile, M., Ceriani, F., Malandra, R., Arioli, F., & Panseri, S. (2019). Risk characterisation from the presence of environmental contaminants and antibiotic residues in wild and farmed salmon from different FAO zones. Food Additives and Contaminants - Part A Chemistry, Analysis, Control, Exposure and Risk Assessment, 36(1), 152–162. https://doi.org/10.1080/19440049.2018.1563723
Commission Regulation (EU) Commission Regulation (EU) No 37/2010 of 22 December 2009 on Pharmacologically Active Substances and Their Classification Regarding Maximum Residue Limits in Foodstuffs of Animal Origin. OJEU L 15/1–72, 20.1.2010.
Collignon, P. J., & McEwen, S. A. (2019). One health-its importance in helping to better control antimicrobial resistance. Tropical Medicine and Infectious Disease, 4(1). https://doi.org/10.3390/tropicalmed4010022
Dinh, Q. T., Munoz, G., Duy, S. V., Do, D. T., Bayen, S., & Sauvé, S. (2020). Analysis of sulfonamides, fluoroquinolones, tetracyclines, triphenylmethane dyes and other veterinary drug residues in cultured and wild seafood sold in Montreal, Canada. Journal of Food Composition and Analysis, 94, 103630. https://doi.org/10.1016/j.jfca.2020.103630
European Council (2001a). Directive 2001/82/EC of the European Parliament and of the Council of 6th November 2001 on the Community code relating to veterinary medicinal products. Off. J. Eur. Community.
European Medicines Agency (EMA) (2022). Maximum Residue Limits (MRL). https://www.ema.europa.eu/en/veterinary-regulatory/research-development/maximum-residue-limits-mrl
Ferri, G., Lauteri, C., & Vergara, A. (2022). Antibiotic Resistance in the Finfish Aquaculture Industry: A Review. Antibiotics, 11(11), 1574. https://doi.org/10.3390/antibiotics11111574
Fu, L., Huang, T., Wang, S., Wang, X., Su, L., Li, C., & Zhao, Y. (2017). Toxicity of 13 different antibiotics towards freshwater green algae Pseudokirchneriella subcapitata and their modes of action. Chemosphere, 168, 217-222. https://doi.org/10.1016/j.chemosphere.2016.10.043
Gamperl, A. K., Ajiboye, O. O., Zanuzzo, F. S., Sandrelli, R. M., Ellen de Fátima, C. P., & Beemelmanns, A. (2020). The impacts of increasing temperature and moderate hypoxia on the production characteristics, cardiac morphology and haematology of Atlantic Salmon (Salmo salar). Aquaculture, 519, 734874. https://doi.org/10.1016/j.aquaculture.2019.734874
Gazal, L. E. de S., Brito, K. C. T. de, Kobayashi, R. K. T., Nakazato, G., Cavalli, L. S., Otutumi, L. K., & Brito, B. G. de. (2020). Antimicrobials and resistant bacteria in global fish farming and the possible risk for public health. Arquivos Do Instituto Biológico, 87, 1–11. https://doi.org/10.1590/1808-1657000362019
Graham, F., Paradis, L., Bégin, P., Paradis, J., Babin, Y., & Des Roches, A. (2014). Risk of allergic reaction and sensitization to antibiotics in foods. Annals of Allergy, Asthma and Immunology, 113(3), 329–330. https://doi.org/10.1016/j.anai.2014.06.029
Grave, K., Markestad, A., & Bangen, M. (1996). Comparison in prescribing patterns of antibacterial drugs in salmonid farming in Norway during the periods 1980‐1988 and 1989‐1994. Journal of veterinary pharmacology and therapeutics, 19(3), 184-191. https://doi.org/10.1111/j.1365-2885.1996.tb00037.x
Grave, K., Hansen, M. K., Kruse, H., Bangen, M., & Kristoffersen, A. B. (2008). Prescription of antimicrobial drugs in Norwegian aquaculture with an emphasis on “new” fish species. Preventive Veterinary Medicine, 83(2), 156-169. https://doi.org/10.1016/j.prevetmed.2007.07.002
Griboff, J., Carrizo, J. C., Bonansea, R. I., Valdés, M. E., Wunderlin, D. A., & Amé, M. V. 2020. Multiantibiotic residues in commercial fish from Argentina. The presence of mixtures of antibiotics in edible fish, a challenge to health risk assessment. Food Chemistry, 332, 127380. https://doi.org/10.1016/j.foodchem.2020.127380
Guidi, L. R., Santos, F. A., Ribeiro, A. C. S., Fernandes, C., Silva, L. H., & Gloria, M. B. A. (2018). Quinolones and tetracyclines in aquaculture fish by a simple and rapid LC-MS/MS method. Food chemistry, 245, 1232-1238. https://doi.org/10.1016/j.foodchem.2017.11.094
Happold, J., Meyer, A., Sadler, R., Cowled, B., Mackenzie, C., Stevenson, M., Ward, M. P., Gallardo Lagno, A. L., & Cameron, A. (2020). Effectiveness of antimicrobial treatment of salmonid rickettsial septicaemia in commercial salmon and trout farms in Chile. Aquaculture, 525, 735323. https://doi.org/10.1016/j.aquaculture.2020.735323
Hemamalini, N., Shanmugam, S. A., Kathirvelpandian, A., Deepak, A., Kaliyamurthi, V., & Suresh, E. (2021). A critical review on the antimicrobial resistance, antibiotic residue and metagenomics‐assisted antimicrobial resistance gene detection in freshwater aquaculture environment. Aquaculture Research, 53(2), 344–366. https://doi.org/10.1111/are.15601
Higuera-Llantén, S., Vásquez-Ponce, F., Barrientos-Espinoza, B., Mardones, F. O., Marshall, S. H., & Olivares-Pacheco, J. (2018). Extended antibiotic treatment in salmon farms select multiresistant gut bacteria with a high prevalence of antibiotic resistance genes. PLoS ONE, 13(9), 1–22. https://doi.org/10.1371/journal.pone.0203641
Horsberg, T. E., Hoff, K. A., & Nordmo, R. (1996). Pharmacokinetics of florfenicol and its metabolite florfenicol amine in Atlantic salmon. Journal of Aquatic Animal Health, 8(4), 292-301. https://doi.org/10.1577/1548-8667(1996)008<0292:POFAIM>2.3.CO;2
Ibrahim, M., Ahmad, F., Yaqub, B., Ramzan, A., Imran, A., Afzaal, M., Mirza, S. A., Mazhar, I., Younus, M., Akram, Q., Ali Taseer, M. S., Ahmad, A., & Ahmed, S. (2020). Current trends of antimicrobials used in food animals and aquaculture. Antibiotics and Antimicrobial Resistance Genes in the Environment, 39–69. https://doi.org/10.1016/b978-0-12-818882-8.00004-8
Jara, B., Tucca, F., Srain, B. M., Méjanelle, L., Aranda, M., Fernández, C., & Pantoja-Gutiérrez, S. (2021). Antibiotics florfenicol and flumequine in the water column and sediments of Puyuhuapi Fjord, Chilean Patagonia. Chemosphere, 275. https://doi.org/10.1016/j.chemosphere.2021.130029
Jara, B., Srain, B. M., Aranda, M., Fernández, C., Pantoja-Gutiérrez, S., & Méjanelle, L. (2022). Water-sediment partitioning of flumequine and florfenicol, two antibiotics used in salmon aquaculture in Chile. Marine Pollution Bulletin, 177, 113480. https://doi.org/10.1016/j.marpolbul.2022.113480
Kumar, M., Jaiswal, S., Sodhi, K. K., Shree, P., Singh, D. K., Agrawal, P. K., & Shukla, P. (2019). Antibiotics bioremediation: Perspectives on its ecotoxicity and resistance. Environment International, 124, 448–461. https://doi.org/10.1016/j.envint.2018.12.065
Leung, K. C., Huang, Q., St-Hilaire, S., Liu, H., Zheng, X., Cheung, K. B., & Zwetsloot, I. M. (2020). Fraudulent antibiotic products on the market for aquaculture use. Preventive Veterinary Medicine, 181, 105052. https://doi.org/10.1016/j.prevetmed.2020.105052
Liu, X., Steele, J. C., & Meng, X. Z. (2017). Usage, residue, and human health risk of antibiotics in Chinese aquaculture: A review. Environmental Pollution, 223, 161–169. https://doi.org/10.1016/j.envpol.2017.01.003
Love, D. C., Fry, J. P., Cabello, F., Good, C. M., & Lunestad, B. T. (2020). Veterinary drug use in United States net pen Salmon aquaculture: Implications for drug use policy. Aquaculture, 518, 734820. https://doi.org/10.1016/j.aquaculture.2019.734820
Lozano-Muñoz, I., Wacyk, J., Kretschmer, C., Vásquez-Martínez, Y., & Martin, M. C. S. (2021). Antimicrobial resistance in Chilean marine-farmed salmon: Improving food safety through One Health. One Health, 12 October 2020. https://doi.org/10.1016/j.onehlt.2021.100219
Lulijwa, R., Rupia, E. J., & Alfaro, A. C. (2020). Antibiotic use in aquaculture, policies and regulation, health and environmental risks: a review of the top 15 major producers. Reviews in Aquaculture, 12(2), 640–663. https://doi.org/10.1111/raq.12344
Luthman, O., Jonell, M., & Troell, M. (2019). Governing the salmon farming industry: Comparison between national regulations and the ASC salmon standard. Marine Policy, 106(April), 103534. https://doi.org/10.1016/j.marpol.2019.103534
Luu, Q. H., Nguyen, T. B. T., Nguyen, T. L. A., Do, T. T. T., Dao, T. H. T., & Padungtod, P. (2021). Antibiotics use in fish and shrimp farms in Vietnam. Aquaculture Reports, 20, 100711. https://doi.org/10.1016/j.aqrep.2021.100711
Maki, T., Hasegawa, H., Kitami, H., Fumoto, K., Munekage, Y., & Ueda, K. (2006). Bacterial degradation of antibiotic residues in marine fish farm sediments of Uranouchi Bay and phylogenetic analysis of antibiotic-degrading bacteria using 16S rDNA sequences. Fisheries Science, 72(4), 811–820. https://doi.org/10.1111/j.1444-2906.2006.01222.x
Menkem, Z. E., Ngangom, B. L., Tamunjoh, S. S. A., & Boyom, F. F. (2019). Antibiotic residues in food animals: Public health concern. Acta Ecologica Sinica, 39(5), 411–415. https://doi.org/10.1016/j.chnaes.2018.10.004
Millanao, A. R., Barrientos-Schaffeld, C., Siegel-Tike, C. D., Tomova, A., Ivanova, L., Godfrey, H. P., Dölz, H. J., Buschmann, A. H., & Cabello, F. C. (2018). Resistencia a los antimicrobianos en Chile y el paradigma de Una Salud: manejando los riesgos para la salud pública humana y animal resultante del uso de antimicrobianos en la acuicultura del salmón y en medicina. Revista Chilena de Infectología, 35(3), 299–308. https://doi.org/10.4067/s0716-10182018000300299
Miranda, C. D., Godoy, F. A., & Lee, M. R. (2018). Current status of the use of antibiotics and the antimicrobial resistance in the Chilean salmon farms. Frontiers in microbiology, 9, 1284. https://doi.org/10.3389/fmicb.2018.01284
Miranda, C. D., & Rojas, R. (2007). Occurrence of florfenicol resistance in bacteria associated with two Chilean salmon farms with different history of antibacterial usage. Aquaculture, 266(1-4), 39-46. https://doi.org/10.1016/j.aquaculture.2007.02.007
Miranda, C. D., & Zemelman, R. (2002). Bacterial resistance to oxytetracycline in Chilean salmon farming. Aquaculture, 212(1-4), 31-47. https://doi.org/10.1016/S0044-8486(02)00124-2
Mo, W. Y., Chen, Z., Leung, H. M., & Leung, A. O. W. (2017). Application of veterinary antibiotics in China’s aquaculture industry and their potential human health risks. Environmental Science and Pollution Research, 24(10), 8978–8989. https://doi.org/10.1007/s11356-015-5607-z
Mog, M., Waikhom, D., Panda, S. P., Sharma, S., Ngasotter, S., Tesia, S., & Varshney, S. (2020). Problems of antibiotic resistance associated with oxytetracycline use in aquaculture: A review. Journal of Entomology and Zoology Studies, 8(3), 1075–1082. http://www.entomoljournal.com
Monteiro, S. H., Garcia, F., Gozi, K. S., Romera, D. M., Francisco, J. G., Moura-Andrade, G. C. R., & Tornisielo, V. L. (2016). Relationship between antibiotic residues and occurrence of resistant bacteria in Nile tilapia (Oreochromis niloticus) cultured in cage-farm. Journal of Environmental Science and Health - Part B Pesticides, Food Contaminants, and Agricultural Wastes, 51(12), 817–823. https://doi.org/10.1080/03601234.2016.1208457
Morrison, D. B., & Saksida, S. (2013). Trends in antimicrobial use in Marine Harvest Canada farmed salmon production in British Columbia (2003-2011). The Canadian veterinary journal = La revue veterinaire canadienne, 54(12), 1160–1163.
Nagaraju, T. V., Sunil, B. M., Chaudhary, B. (2022). Un estudio sobre el transporte de lixiviados de residuos acuícolas a través del suelo. En Tendencias recientes en ingeniería civil: actas seleccionadas de ICRACE 2021. https://doi.org/10.1007/978-981-19-4055-2_39
Nakano, T., Hayashi, S., & Nagamine, N. (2018). Effect of excessive doses of oxytetracycline on stress-related biomarker expression in coho salmon. Environmental Science and Pollution Research, 25(8), 7121–7128. https://doi.org/10.1007/s11356-015-4898-4
Neis, B., Gao, W., Cavalli, L., Thorvaldsen, T., Holmen, I. M., Jeebhay, M. F., & Tapia-Jopia, C. (2023). Mass mortality events in marine salmon aquaculture and their influence on occupational health and safety hazards and risk of injury. Aquaculture, 739225. https://doi.org/10.1016/j.aquaculture.2022.739225
Nguyen, C. C., Hugie, C. N., Kile, M. L., & Navab-Daneshmand, T. (2019). Association between heavy metals and antibiotic-resistant human pathogens in environmental reservoirs: A review. Frontiers of Environmental Science and Engineering, (133). https://doi.org/10.1007/s11783-019-1129-0
Norambuena-Subiabre, L., González, M. P., & Contreras-Lynch, S. (2018). Oxytetracycline depletion and withdrawal time estimation following intraperitoneal administration in three species from Chilean salmon farming. Aquaculture Research, 49(2), 593–602. https://doi.org/10.1111/are.13501
Nya, E. J., & Austin, B. (2009). Use of garlic, Allium sativum, to control Aeromonas hydrophila infection in rainbow trout, Oncorhynchus mykiss (Walbaum). Journal of fish diseases, 32(11), 963-970. https://doi.org/10.1111/j.1365-2761.2009.01100.x
Oberlé, K., Bouju‐Albert, A., Helsens, N., Pangga, G., Prevost, H., Magras, C., & Calvez, S. (2022). No evidence for a relationship between farm or transformation process locations and antibiotic resistance patterns of Pseudomonas population associated with rainbow trout (Oncorhynchus mykiss). Journal of Applied Microbiology, 132(3), 1738-1750. https://doi.org/10.1111/jam.15344
Ojasanya, R. A., Gardner, I. A., Groman, D. B., Saksida, S., Saab, M. E., & Thakur, K. K. (2022). Antimicrobial susceptibility profiles of bacteria commonly isolated from farmed salmonids in Atlantic Canada (2000 – 2021). Veterinary sciences, 9(4), 159. https://doi.org/10.3390/vetsci9040159
Okocha, R. C., Olatoye, I. O., & Adedeji, O. B. (2018). Food safety impacts of antimicrobial use and their residues in aquaculture. Public Health Reviews, 39(1), 1–22. https://doi.org/10.1186/s40985-018-0099-2
Opstad, L., Idsø, J., & Valenta, R. (2022). The Dynamics of Profitability among Salmon Farmers—A Highly Volatile and Highly Profitable Sector. Fishes, 7(3), 1–15. https://doi.org/10.3390/fishes7030101
Ortiz, P., Quiroga, E., Montero, P., Hamame, M., & Betti, F. (2021). Trophic structure of benthic communities in a Chilean fjord (45° S) influenced by salmon aquaculture: Insights from stable isotopic signatures. Marine Pollution Bulletin, 173, 113149. https://doi.org/10.1016/j.marpolbul.2021.113149
Payne, C. J., Turnbull, J. F., MacKenzie, S., & Crumlish, M. (2022). The effect of oxytetracycline treatment on the gut microbiome community dynamics in rainbow trout (Oncorhynchus mykiss) over time. Aquaculture, 560, 738559. https://doi.org/10.1016/j.aquaculture.2022.738559
Pepi, M., & Focardi, S. (2021). Antibiotic-resistant bacteria in aquaculture and climate change: A challenge for health in the mediterranean area. International Journal of Environmental Research and Public Health, 18(11). https://doi.org/10.3390/ijerph18115723
Poblete, E. G., Drakeford, B. M., Ferreira, F. H., Barraza, M. G., & Failler, P. (2019). The impact of trade and markets on Chilean Atlantic salmon farming. Aquaculture International, 27(5), 1465–1483. https://doi.org/10.1007/s10499-019-00400-7
Preena, P. G., Swaminathan, T. R., Kumar, V. J. R., & Singh, I. S. B. (2020). Antimicrobial resistance in aquaculture: a crisis for concern. Biologia, 75(9), 1497–1517. https://doi.org/10.2478/s11756-020-00456-4
Price, D., Sánchez, J., Ibarra, R., & St-Hilaire, S. (2019). Variation in the concentration of antibiotics in tissue during oral antibiotic treatments in farmed salmonids. Aquaculture, 498, 587–593. https://doi.org/10.1016/j.aquaculture.2018.09.001
Price, D., Sánchez, J., McClure, J., McConkey, S., Ibarra, R., & St-Hilaire, S. (2018). Assessing concentration of antibiotics in tissue during oral treatments against piscirickettsiosis. Preventive Veterinary Medicine, 156, 16–21. https://doi.org/10.1016/j.prevetmed.2018.04.014
Quiñones, R. A., Fuentes, M., Montes, R. M., Soto, D., & León-Muñoz, J. (2019). Environmental issues in Chilean salmon farming: a review. Reviews in Aquaculture, 11(2), 375–402. https://doi.org/10.1111/raq.12337
Rairat, T., Chen, S. M., Lu, Y. P., Hsu, J. C. N., Liu, Y. K., & Chou, C. C. (2022). Determination of temperature-dependent optimal oral doses of florfenicol and corresponding withdrawal times in Nile tilapia (Oreochromis niloticus) reared at 25 and 30° C. Aquaculture, 561, 738719. https://doi.org/10.1016/j.aquaculture.2022.738719
Ramírez, C., Gutiérrez, M. S., Venegas, L., Sapag, C., Araya, C., Caruffo, M., López, P., Reyes-Jara, A., Toro, M., González-Rocha, G., Yáñez, J. M., & Navarrete, P. (2022). Microbiota composition and susceptibility to florfenicol and oxytetracycline of bacterial isolates from mussels (Mytilus spp.) reared on different years and distance from salmon farms. Environmental Research, 204. https://doi.org/10.1016/j.envres.2021.112068
Refstie, S., Bakke-McKellep, A. M., Penn, M. H., Sundby, A., Shearer, K. D., & Krogdahl, Å. (2006). Capacity for digestive hydrolysis and amino acid absorption in Atlantic salmon (Salmo salar) fed diets with soybean meal or inulin with or without addition of antibiotics. Aquaculture, 261(1), 392-406. https://doi.org/10.1016/j.aquaculture.2006.08.005
Reverter, M., Sarter, S., Caruso, D., Avarre, J. C., Combe, M., Pepey, E., Pouyaud, L., Vega-Heredía, S., de Verdal, H., & Gozlan, R. E. (2020). Aquaculture at the crossroads of global warming and antimicrobial resistance. Nature Communications, 11(1), 1–8. https://doi.org/10.1038/s41467-020-15735-6
Rigos, G., Kogiannou, D., Padrós, F., Cristofol, C., Florio, D., Fioravanti, M., & Zarza, C. (2021). Best therapeutic practices for the use of antibacterial agents in finfish aquaculture: A particular view on European seabass (Dicentrarchus labrax) and gilthead seabream (Sparus aurata) in Mediterranean aquaculture. Reviews in aquaculture, 13(3), 1285-1323. https://doi.org/10.1111/raq.12523
Saglam, N., & Yonar, M. E. 2009. Effects of sulfamerazine on selected haematological and immunological parameters in rainbow trout (Onchorhynchus mykiss, Walbaum, 1792). Aquaculture Research, 40(4), 395-404. https://doi.org/10.1111/j.1365-2109.2008.02105.x
Salomón, R., Furones, M. D., Reyes-López, F. E., Tort, L., Firmino, J. P., et al. (2021). A bioactive extract rich in triterpenic acid and polyphenols from olea europaea promotes systemic immunity and protects Atlantic salmon smolts against furunculosis. Frontiers in Immunology, 12, 737601.
San Martin, B., Fresno, M., Maddaleno, A., Burgos, J. M., Anadón, A., Zavala, S., Lapierre, L., Pokrant, E., & Cornejo, J. (2021). Depletion of oxytetracycline plus epi-oxytetracycline residues in rainbow trout (Oncorhynchus mykiss) and Atlantic salmon (Salmo salar) under field conditions in Chile. Aquaculture, 545. https://doi.org/10.1016/j.aquaculture.2021.737154
Santos, L., & Ramos, F. (2018). Antimicrobial resistance in aquaculture: Current knowledge and alternatives to tackle the problem. International Journal of Antimicrobial Agents, 52(2), 135–143. https://doi.org/10.1016/j.ijantimicag.2018.03.010
Schar, D., Klein, E. Y., Laxminarayan, R., Gilbert, M., & Van Boeckel, T. P. (2020). Global trends in antimicrobial use in aquaculture. Scientific Reports, 10(1), 1–9. https://doi.org/10.1038/s41598-020-78849-3
Serwecinska, L. (2020). Antimicrobials and Antibiotic-Resistant Bacteria: A risk to the Environment and to Public Health. Water, 12(12), 3313. https://doi.org/10.3390/w12123313
Shen, X., Jin, G., Zhao, Y., & Shao, X. (2020). Prevalence and distribution analysis of antibiotic resistance genes in a large-scale aquaculture environment. Science of the Total Environment, 711, 134626. https://doi.org/10.1016/j.scitotenv.2019.134626
Slimestad, R., Johny, A., Thomsen, M. G., Karlsen, C. R., & Rosnes, J. T. (2022). Chemical Profiling and Biological Activity of Extracts from Nine Norwegian Medicinal and Aromatic Plants. Molecules, 27(21), 7335. https://doi.org/10.3390/molecules27217335
Smith, P. (2008). Antimicrobial resistance in aquaculture. Revue scientifique et technique (International Office of Epizootics), 27(1), 243-264.
Sobral, M. M. C., Cunha, S. C., Faria, M. A., & Ferreira, I. M. P. L. V. O. (2018). Domestic Cooking of Muscle Foods: Impact on Composition of Nutrients and Contaminants. Comprehensive Reviews in Food Science and Food Safety, 17(2), 309–333. https://doi.org/10.1111/1541-4337.12327
Thiang, E. L., Lee, C. W., Takada, H., Seki, K., Takei, A., Suzuki, S., Wang, A., & Bong, C. W. (2021). Antibiotic residues from aquaculture farms and their ecological risks in Southeast Asia: a case study from Malaysia. Ecosystem Health and Sustainability, 7(1). https://doi.org/10.1080/20964129.2021.1926337
Thomassen, G. M. B., Reiche, T., Tennfjord, C. E., & Mehli, L. (2022). Antibiotic Resistance Properties among Pseudomonas spp. Associated with Salmon Processing Environments. Microorganisms, 10(7), 1420. https://doi.org/10.3390/microorganisms10071420
Tilseth, S., Hansen, T., & Møller, D. (1991). Historical development of salmon culture. Aquaculture, 98(1-3), 1-9. https://doi.org/10.1016/0044-8486(91)90367-G
Treiber, F. M., & Beranek-Knauer, H. (2021). Antimicrobial residues in food from animal origin—a review of the literature focusing on products collected in stores and markets worldwide. Antibiotics, 10(5). https://doi.org/10.3390/antibiotics10050534
Trueman, J. D., Filgueira, R., & Fanning, L. (2022). Transparency and communication in Norwegian and Nova Scotian Atlantic salmon aquaculture industries. Marine Policy, 138. https://doi.org/10.1016/j.marpol.2022.104958
United Stated Department of Agriculture (USDA). (2022). Maximum Residue Limits (MRL) Database. https://www.fas.usda.gov/maximum-residue-limits-mrl-database.
Vilca, F. Z., Galarza, N. C., Tejedo, J. R., Cuba, W. A. Z., Quiróz, C. N. C., & Tornisielo, V. L. (2021). Occurrence of residues of veterinary antibiotics in water, sediment and trout tissue (Oncorhynchus mykiss) in the southern area of Lake Titicaca, Peru. Journal of Great Lakes Research, 47(4), 1219-1227. https://doi.org/10.1016/j.jglr.2021.04.012
Vincent, A. T., Gauthier, J., Derome, N., & Charette, S. J. (2019). The Rise and Fall of Antibiotics in Aquaculture. Microbial Communities in Aquaculture Ecosystems, 1–19. https://doi.org/10.1007/978-3-030-16190-3_1
Wang, F., Lu, Y., & Cao, J. (2022). Dynamics impacts of oxytetracycline on growth performance, intestinal health and antibiotic residue of grouper in exposure and withdrawal treatment. Ecotoxicology and Environmental Safety, 247, 114203. https://doi.org/10.1016/j.ecoenv.2022.114203
Watts, J. E. M., Schreier, H. J., Lanska, L., & Hale, M. S. (2017). The rising tide of antimicrobial resistance in aquaculture: Sources, sinks and solutions. Marine Drugs, 15(6), 1–16. https://doi.org/10.3390/md15060158
Wilson, B. A., Smith, V. H., deNoyelles, F., & Larive, C. K. (2003). Effects of three pharmaceutical and personal care products on natural freshwater algal assemblages. Environmental science & technology, 37(9), 1713-1719. https://doi.org/10.1021/es0259741
Xu, N., Cheng, B., Li, M., Lin, Z., & Ai, X. (2021). Withdrawal interval estimation of doxycycline in yellow catfish (Pelteobagrus fulvidraco) using an lc-ms/ms method based upon quechers sampling preparation. Foods, 10(11). https://doi.org/10.3390/foods10112554
Yang, F., Yang, F., Wang, G., Kong, T., Wang, H., & Zhang, C. (2020). Effects of water temperature on tissue depletion of florfenicol and its metabolite florfenicol amine in crucian carp (Carassius auratus gibelio) following multiple oral doses. Aquaculture, 515, 734542. https://doi.org/10.1016/j.aquaculture.2019.734542
Yang, F., Yang, F., Wang, D., Zhang, C.-S., Wang, H., Song, Z.-W., Shao, H.-T., Zhang, M., Yu, M.-L., & Zheng, Y. (2021). Development and Application of a Water Temperature Related Physiologically Based Pharmacokinetic Model for Enrofloxacin and Its Metabolite Ciprofloxacin in Rainbow Trout. Frontiers in Veterinary Science, 7. https://doi.org/10.3389/fvets.2020.608348
Yonar, M. E. (2012). The effect of lycopene on oxytetracycline-induced oxidative stress and immunosuppression in rainbow trout (Oncorhynchus mykiss, W.). Fish & shellfish immunology, 32(6), 994-1001. https://doi.org/10.1016/j.fsi.2012.02.012
Yuan, J., Ni, M., Liu, M., Zheng, Y., & Gu, Z. (2019). Occurrence of antibiotics and antibiotic resistance genes in a typical estuary aquaculture region of Hangzhou Bay, China. Marine Pollution Bulletin, 138, 376–384. https://doi.org/10.1016/j.marpolbul.2018.11.037
Zanuzzo, F. S., Peroni, E. de F. C., Sandrelli, R. M., St-Hilaire, S., O’Brien, N., & Gamperl, A. K. (2022). Temperature has considerable effects on plasma and muscle antibiotic concentrations in Atlantic salmon (Salmo salar). Aquaculture, 546. https://doi.org/10.1016/j.aquaculture.(2021).737372
Zhou, L. J., Wu, Q. L., Zhang, B. B., Zhao, Y. G., & Zhao, B. Y. (2016). Occurrence, spatiotemporal distribution, mass balance and ecological risks of antibiotics in subtropical shallow Lake Taihu, China. Environmental Science: Processes & Impacts, 18(4), 500-513. https://doi.org/10.1039/C6EM00062B
Publicado
Cómo citar
Número
Sección
Licencia
Derechos de autor 2024 Scientia Agropecuaria

Esta obra está bajo una licencia internacional Creative Commons Atribución-NoComercial 4.0.
Los autores que publican en esta revista aceptan los siguientes términos:
a. Los autores conservan los derechos de autor y conceden a la revista el derecho publicación, simultáneamente licenciada bajo una licencia de Creative Commons que permite a otros compartir el trabajo, pero citando la publicación inicial en esta revista.
b. Los autores pueden celebrar acuerdos contractuales adicionales separados para la distribución no exclusiva de la versión publicada de la obra de la revista (por ejemplo, publicarla en un repositorio institucional o publicarla en un libro), pero citando la publicación inicial en esta revista.
c. Se permite y anima a los autores a publicar su trabajo en línea (por ejemplo, en repositorios institucionales o en su sitio web) antes y durante el proceso de presentación, ya que puede conducir a intercambios productivos, así como una mayor citación del trabajo publicado (ver efecto del acceso abierto).




