An effective disinfection protocol for contamination control in vitro establishment of Mortiño (Vaccinium floribundum Kunth) and identification of endogenous microbes
DOI:
https://doi.org/10.17268/sci.agropecu.2025.028Palabras clave:
Vaccinium floribundum Kunth, micropropagation, endogenous contamination, Mortiño, Andean blueberryResumen
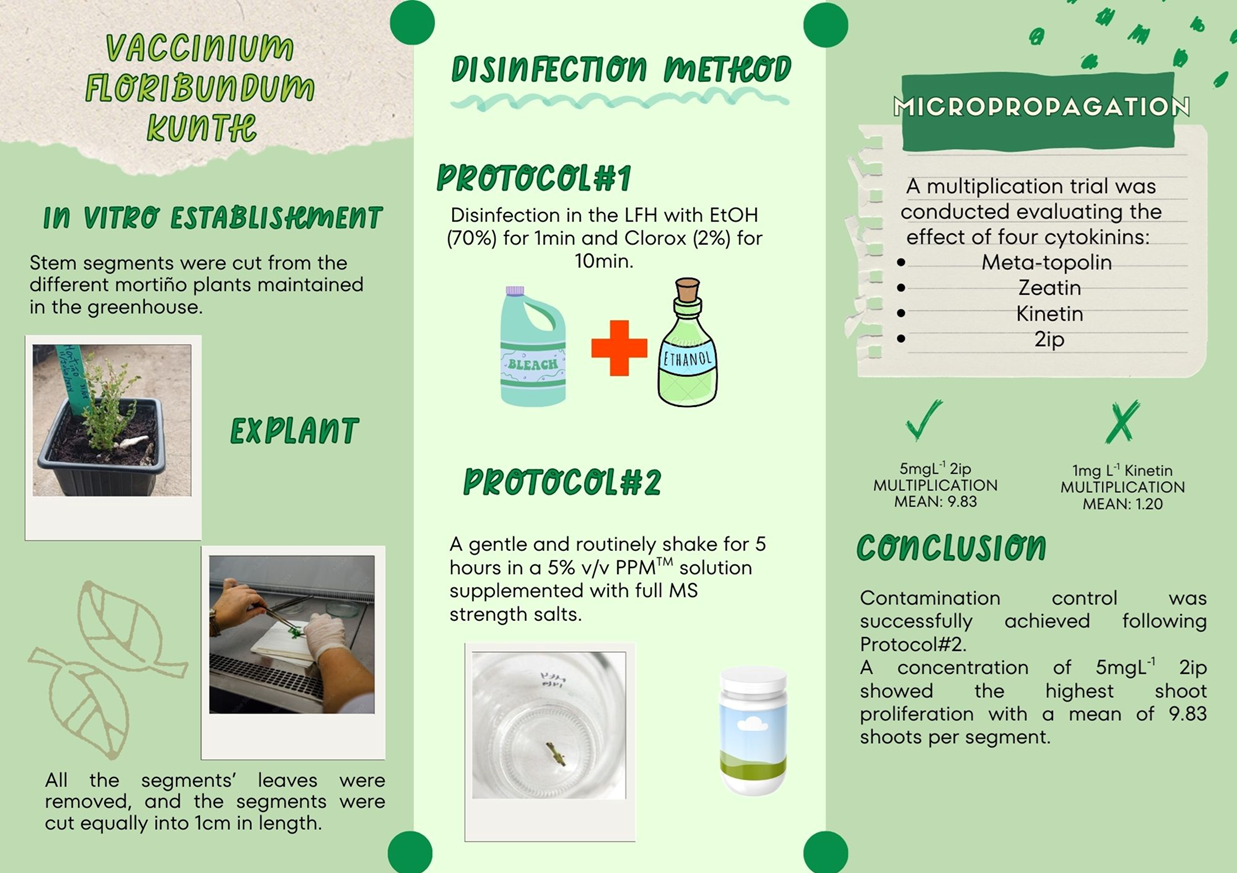
The Vaccinium genus consists of a variety of berries with high nutritious components consumed worldwide leading to the development of micropropagation protocols to supply the demand. Mortiño, the Andean Blueberry (Vaccinium floribundum Kunth) is a wild berry that grows in high-altitude grasslands with nutritious and commercial potential in Ecuador. In this study, the use of PPMTM (Plant Preservative Mixture™) was effective controlling contamination for the in vitro establishment of Vaccinium floribundum Kunth in contrast to a conventional method using EtOH and Clorox. Stems were defoliated and cut into 1 cm segments, then immersed in liquid MS (Murashige & Skoog) supplemented with 5% v/v PPMTM without pH adjustment for 5 hours under constant shaking. After immersion, segments were transferred to flasks containing WPM (Woody Plant Media) medium supplemented with an additional 2 mlL-1 PPMTM. Persistent microbial contaminants in the in vitro explants were isolated and identified through molecular methods and gene sequences analyzed using the GenBank database resulted in the identification of three bacterial species: Methylobacterium sp., Methylobacterium radiotolerans, and Bacillus pumilus. In addition, three fungal species were also discovered: Xylaria sp., Xylaria feejeensis, and Diaporthe lutecens. Additionally, a multiplication assay was made with the aseptic stems from the sterilization protocol to evaluate four different growth regulators: 2ip, kinetin, zeatin and meta-topolin. kinetin showed very low responses with a mean of 1.2 shoots per stem. The highest number of shoots per stem (9 shoots) was obtained with 5 mg L-1 2ip. The use of zeatin and meta-topolin facilitated shoot proliferation with the following concentrations: 3 mg L-1 zeatin + 0.5 mg L-1 NAA (1-Naphthaleneacetic Acid) and 3 mg L-1 Meta-topolin + 0.5 mg L-1 NAA. These findings demonstrate the successful establishment of an in vitro disinfection and multiplication protocol for V. floribundum.
Citas
Abdalla, N., El-Ramady, H., Seliem, M. K., El-Mahrouk, M. E., Taha, N., Bayoumi, Y., ... & Dobránszki, J. (2022). An academic and technical overview on plant micropropagation challenges. Horticulturae, 8(8), 677. https://doi.org/10.3390/horticulturae8080677
Aleynova, O. A., & Kiselev, K. V. (2023). Interaction of plants and endophytic microorganisms: molecular aspects, biological functions, community composition, and practical applications. Plants, 12(4), 714. https://doi.org/10.3390/plants12040714
Baba, T., Hirose, D., Sasaki, N., Watanabe, N., Kobayashi, N., Kurashige, Y., Karimi, F., & Ban, T. (2016). Mycorrhizal formation and diversity of endophytic fungi in hair roots of Vaccinium oldhamii Miq. in Japan. Microbes and Environments, 31(2), 186–189. https://doi.org/10.1264/jsme2.ME16011
Barrera, A., Hereme, R., Ruiz-Lara, S., Larrondo, L. F., Gundel, P. E., Pollmann, S., Molina-Montenegro, M. A., & Ramos, P. (2020). Fungal endophytes enhance the photoprotective mechanisms and photochemical efficiency in the Antarctic Colobanthus quitensis (Kunth) Bartl. exposed to UV-B radiation. Frontiers in Ecology and Evolution, 8. https://doi.org/10.3389/fevo.2020.00122
Brooks, S., Klomchit, A., Chimthai, S., Jaidee, W., & Bastian, A. C. (2022). Xylaria feejeensis, SRNE2BP a fungal endophyte with biocontrol properties to control early blight and Fusarium wilt disease in tomato and plant growth promotion activity. Current Microbiology, 79(4), 108. https://doi.org/10.1007/s00284-022-02803-x
Cappelletti, R., & Mezzetti, B. (2014, August). TDZ, 2iP and zeatin in blueberry (Vaccinium corymbosum L.'Duke') in vitro proliferation and organogenesis. In XXIX International Horticultural Congress on Horticulture: Sustaining Lives, Livelihoods and Landscapes
Cobo, M. M., Gutiérrez, B., & de Lourdes Torres, M. (2018). Regeneration of mortiño (Vaccinium floribundum Kunth) plants through axillary bud culture. İn vitro cellular & developmental biology-plant, 54, 112-116. https://doi.org/10.1007/s11627-018-9884-3
Compton, M. E., & Koch, J. M. (2001). Influence of plant preservative mixture (PPM)TM on adventitious organogenesis in melon, petunia, and tobacco. In Vitro Cell.Dev.Biol.-Plant, 37, 259–261. https://doi.org/10.1007/s11627-001-0046-6
Cordovana, M., Deni, A., Kostrzewa, M., Abdalla, M., & Ambretti, S. (2019). First report of Methylobacterium radiotolerans bacteraemia identified by MALDI-TOF mass spectrometry. In New Microbes and New Infections (Vol. 30). Elsevier Ltd. https://doi.org/10.1016/j.nmni.2019.100546
Cruz, M., & Lasso, E. (2021). Insights into the functional ecology of páramo plants in Colombia. Biotropica, 53(5), 1415-1431. https://doi.org/10.1111/btp.12992
Debnath, S. C. (2008). Zeatin-induced one-step in vitro cloning affects the vegetative growth of cranberry (Vaccinium macrocarpon Ait.) micropropagules over stem cuttings. Plant cell, tissue and organ culture, 93(2), 231-240. https://doi.org/10.1007/s11240-008-9366-0
Debnath, S. C., & McRae, K. B. (2001). In vitro culture of lingonberry (Vaccinium vitis-idaea L.) the influence of cytokinins and media types on propagation. Small Fruits Review, 1(3), 3-19. https://doi.org/10.1300/J301v01n03_02
Dudeja, S. S., Suneja-Madan, P., Paul, M., Maheswari, R., & Kothe, E. (2021). Bacterial endophytes: Molecular interactions with their hosts. In Journal of Basic Microbiology (Vol. 61, Issue 6, pp. 475–505). John Wiley and Sons Inc. https://doi.org/10.1002/jobm.202000657
Ďurkovič, J., & Lux, A. (2010). Micropropagation with a novel pattern of adventitious rooting in American sweetgum (Liquidambar styraciflua L.). Trees, 24, 491-497. https://doi.org/10.1007/s00468-010-0418-9
Dobrzyński, J., Jakubowska, Z., Kulkova, I., Kowalczyk, P., & Kramkowski, K. (2023). Biocontrol of fungal phytopathogens by Bacillus pumilus. In Frontiers in Microbiology (Vol. 14). Frontiers Media SA. https://doi.org/10.3389/fmicb.2023.1194606
Georgieva, M., Kondakova, V., & Yancheva, S. (2016). Micropropagation of high-bush blueberry (Vaccinium corymbosum L.) Toro cultivar. Journal of Pomology, 50, 119-123. Georgieva, M., & Kondakova, V. (2021). In vitro propagation of Vaccinium corymbosum L. Bulgarian Journal of Agricultural Science, 27(2), 323-327.
Gholizadeh, A. (2012). Molecular evidence for the contribution of methylobacteria to the pink-pigmentation process in pink-colored plants. Journal of Plant Interactions, 7(4), 316–321. https://doi.org/10.1080/17429145.2012.693208
Hilário, S., & Gonçalves, M. F. M. (2022). endophytic diaporthe as promising leads for the development of biopesticides and biofertilizers for a sustainable agriculture. Microorganisms, 10(12). https://doi.org/10.3390/microorganisms10122453
Ho, W. J., Huang, Y. K., Huang, W. W., Huang, Y. C., & Chung, J. P. (2022). Effective in vitro culture using dormant bud of nodal sections from a mature Acacia tree. In Vitro Cellular & Developmental Biology-Plant, 58, 437–446. https://doi.org/10.1007/s11627-021-10235-8
Huh, Y. S., Lee, J. K., Kim, I. J., Kang, B. G., & Lee, K. Y. (2015). Effect of biocide addition on plantlet growth and contamination occurrence during the in vitro culture of blueberry. Journal of Plant Biotechnology, 42(2), 111-116. https://doi.org/10.5010/JPB.2015.42.2.111
Aleynova, O. A., & Kiselev, K. V. (2023). Interaction of plants and endophytic microorganisms: Molecular aspects, biological functions, community composition, and practical applications. Plants, 12(4), 714. https://doi.org/10.3390/plants12040714
Jørgensen, P. M., León-Yánez, S., González, A. P., Swift, V. A., Hediger, N. L., & Missouri Botanical Garden, P. H. (1999). Catalogue of the vascular plants of Ecuador= Catálogo de las plantas vasculares del Ecuador. St. Louis, Mo: Missouri Botanical Garden Press. ISBN 0-915297-60-6 ISSN 0161-1542
Jørgensen, P. M. C., Ulloa, R., Valencia, & Madsen, J. E. (1995). A floristic analysis of the high Andes of Ecuador. S.P. Churchill et al. (editors), Biodiversity and Conservation of Neotropical Montane Forest. The New York Botanical Garden, pags. 221–237.
Joshee, N., Biswas, B. K., & Yadav, A. K. (2007). Somatic embryogenesis and plant development in Centella asiatica L., a highly prized medicinal plant of the tropics. HortScience, 42(3), 633-637. https://doi.org/10.21273/HORTSCI.42.3.633
Llerena, W., Samaniego, I., Ramos, M., & Brito, B. (2014). Caracterización Físicoquímica y Funcional de Seis Frutas Tropicales y Andinas Ecuatorianas. Alimentos, Ciencia e Ingeniería, 22(2), 13-22.
Llivisaca, S., Cevallos, J. C., Mendoza, J., Piña, F., Peralta, E., Timm, E. S., & Flores, J. (2020). In vitro Propagation of Mortiño (Vaccinium floribundum Kunth.). Plant Tissue Culture and Biotechnology, 30(2), 167-177. https://doi.org/10.3329/ptcb.v30i2.50687
Leifert, C., Ritchie, J. Y., Waites, W. M. (1991). Contaminants of plant-tissue and cell Cultures. World J Microbiol Biotechnol, 7, 452-469. https://doi.org/10.1007/BF00303371
Leifert, C., Morris, C. E., & Waites, W. M. (1994). In: Ecology of microbial saprophytes and pathogens in tissue culture and field-grown plants: reasons for contamination problems in vitro. Critical Reviews in Plant Sciences, 13(2), 139–183. http://dx.doi.org/10.1080/07352689409701912
Macías-Rubalcava, M. L., & Sánchez-Fernández, R. E. (2017). Secondary metabolites of endophytic Xylaria species with potential applications in medicine and agriculture. World Journal of Microbiology and Biotechnology, 33(1). https://doi.org/10.1007/s11274-016-2174-5
Mahmoud, S. N., & Al-Ani, N. K. (2016). Effect of different sterilization methods on contamination and viability of nodal segments of Cestrum nocturnum L. International Journal of Research Studies in Biosciences, 4(1), 4-9. http://dx.doi.org/10.20431/2349-0365.0401002
McCown, B. H., & Lloyd, G. (1981). Woody Plant Medium (WPM) - A Mineral Nutrient Formulation for Microculture of Woody Plant Species.
Meiners, J., Schwab, M., & Szankowski, I. (2007). Efficient in vitro regeneration systems for Vaccinium species. Plant Cell, Tissue, and Organ Culture, 89, 169-176. https://doi.org/10.1007/s11240-007-9230-7
Medina Cano, C. I., Martínez Bustamante, E., & López Orozco, C. A. (2019). Phenological scale for the mortiño or agraz (Vaccinium meridionale Swartz) in the high Colombian Andean area. Revista Facultad Nacional de Agronomía Medellín, 72(3), 8897-8908. https://doi.org/10.15446/rfnam.v72n3.74460
Meneses, L. S., Morillo, L. E., & Vásquez-Castillo, W. (2022). In vitro propagation of Vaccinium floribundum Kunth from seeds: promissory technology for mortiño accelerated production. Canadian Journal of Plant Science, 102(1), 216-224. https://doi.org/10.1139/cjps-2020-0290
Murashige, T., & Skoog, F. (1962). A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiologia plantarum, 15(3). https://doi.org/10.1111/j.1399-3054.1962.tb08052.x
Muriel, P. (2017). Guatteria microcarpa. Libro Rojo de Plantas Endémicas del Ecuador. Publicaciones del Herbario QCA, Pontificia Universidad Católica del Ecuador, Quito.
Osmundson, T. W., Eyre, C. A., Hayden, K. M., Dhillon, J., & Garbelotto, M. M. (2013). Back to basics: An evaluation of NaOH and alternative rapid DNA extraction protocols for DNA barcoding, genotyping, and disease diagnostics from fungal and oomycete samples. Molecular Ecology Resources, 13(1), 66–74. https://doi.org/10.1111/1755-0998.12031
Palberg, D., Kisiała, A., Jorge, G. L., & Emery, R. J. N. (2022). A survey of Methylobacterium species and strains reveals widespread production and varying profiles of cytokinin phytohormones. BMC Microbiology, 22(1). https://doi.org/10.1186/s12866-022-02454-9
Park, S. (2021). Plant tissue culture: techniques and experiments. Academic Press. Pag 48.
Plant Cell Technology. (2025). Plant Preservative Mixture. https://plantcelltechnology.com/products/plant-upreservative-mixture-ppm
Pedraza-Peñosa, P., Valencia, R., Montúfar, R., Santiana, J., & Tye, A. (2017). Ericaceae. En: León-Yánez, et al. (Eds). Libro Rojo de Plantas Endémicas del Ecuador. Publicaciones del Herbario QCA, Pontificia Universidad Católica del Ecuador, Quito.
R Core Team (2022). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/.
Rani, S., Kumar, P., Dahiya, P., Maheshwari, R., Dang, A. S., & Suneja, P. (2022). Endophytism: A multidimensional approach to plant–prokaryotic microbe interaction. Frontiers in Microbiology, 13. https://doi.org/10.3389/fmicb.2022.861235
Romero-Benavides, J. C., Duarte-Casar, R., Rojas-Le-Fort, M., & Bailon-Moscoso, N. (2025). Colada Morada, A Traditional Ecuadorian Day of the Dead Beverage: A Bibliometric Analysis and Review of the Biological Activity of Native Ecuadorian Ingredients. Journal of Agriculture and Food Research, 19, 101701. https://doi.org/10.1016/j.jafr.2025.101701
Rosenblueth, M., & Martínez-Romero, E. (2006). Bacterial endophytes and their interactions with hosts. Mol Plant Microbe Interact, 19(8), 827–837. https://doi.org/10.1094/mpmi-19-0827
San José, M. C., Cernadas, M. J., & Janeiro, L. V. (2021). Optimization of micropropagation protocols in some woody plants using Meta-topolin. In: Ahmad, N., Strnad, M. (eds) Meta-topolin: A Growth Regulator for Plant Biotechnology and Agriculture. Springer, Singapore. https://doi.org/10.1007/978-981-15-9046-7_16
Santamaría, P. C., Coronel, D., Verdugo, K., Paredes, M. F., Yugsi, E., & Huachi, L. (2012). Estudio etnobotánico del mortiño (Vaccinium floribundum) como alimento ancestral y potencial alimento funcional. LA GRANJA, 16(2), 5-13.
Skrovankova, S., Sumczynski, D., Mlcek, J., Jurikova, T., & Sochor, J. (2015). Bioactive compounds and antioxidant activity in different types of berries. Int. J. Mol. Sci., 16, 24673–24706. https://doi.org/10.3390/ijms161024673
Schuchovski, C. S., & Biasi, L. A. (2019). In vitro establishment of ‘Delite’rabbiteye blueberry microshoots. Horticulturae, 5(1), 24. https://doi.org/10.3390/horticulturae5010024
Torres M., Trujillo D., & Arahana V. S. 2010. Cultivo in vitro del mortiño (Vaccinium floribundum Kunth). ACI Avances en Ciencias e Ingenierías, 2, B9–B15. https://doi.org/10.18272/aci.v2i2.27
Vasco, C., Riihinen, K., Ruales, J., & Kamal-Eldin, A. (2009). Chemical composition and phenolic compound profile of mortiño (Vaccinium floribundum Kunth). Journal of Agricultural and Food Chemistry, 57(18), 8274-8281. https://doi.org/10.1021/jf9013586
Volk, G. M., Bonnart, R., de Oliveira, A. C. A., & Henk, A. D. (2022). Minimizing the deleterious effects of endophytes in plant shoot tip cryopreservation. Applications in Plant Sciences, 10(5), e11489. https://doi.org/10.1002/aps3.11489
Vreugdenhil, D., Bradshaw, J., Gebhardt, C., Govers, F., Taylor, M. A., MacKerron, D. K., & Ross, H. A. (Eds.). (2011). Potato biology and biotechnology: Advances and perspectives. ElSevier.
White, J. F., Kingsley, K. L., Zhang, Q., Verma, R., Obi, N., Dvinskikh, S., Elmore, M. T., Verma, S. K., Gond, S. K., & Kowalski, K. P. (2019). Endophytic microbes and their potential applications in crop management. Pest Management Science, 75(10), 2558–2565. https://doi.org/10.1002/ps.5527
Yang, W. Q., Goulart, B. L., Demchak, K., & Li, Y. (2002). Interactive Effects of mycorrhizal inoculation and organic soil amendments on nitrogen acquisition and growth of highbush blueberry. J. Amer. Soc. Hort. Sci, 127(5), 742–748. https://doi.org/10.21273/JASHS.127.5.742
Yoshida, S., Hiradate, S., Koitabashi, M., Kamo, T., & Tsushima, S. (2017). Phyllosphere Methylobacterium bacteria contain UVA-absorbing compounds. Journal of Photochemistry and Photobiology B: Biology, 167, 168–175. https://doi.org/10.1016/j.jphotobiol.2016.12.019
Descargas
Publicado
Cómo citar
Número
Sección
Licencia
Derechos de autor 2025 Scientia Agropecuaria

Esta obra está bajo una licencia internacional Creative Commons Atribución-NoComercial 4.0.
Los autores que publican en esta revista aceptan los siguientes términos:
a. Los autores conservan los derechos de autor y conceden a la revista el derecho publicación, simultáneamente licenciada bajo una licencia de Creative Commons que permite a otros compartir el trabajo, pero citando la publicación inicial en esta revista.
b. Los autores pueden celebrar acuerdos contractuales adicionales separados para la distribución no exclusiva de la versión publicada de la obra de la revista (por ejemplo, publicarla en un repositorio institucional o publicarla en un libro), pero citando la publicación inicial en esta revista.
c. Se permite y anima a los autores a publicar su trabajo en línea (por ejemplo, en repositorios institucionales o en su sitio web) antes y durante el proceso de presentación, ya que puede conducir a intercambios productivos, así como una mayor citación del trabajo publicado (ver efecto del acceso abierto).




