Antioxidant activity and seed vigor in germination of bean under salt stress conditions
DOI:
https://doi.org/10.17268/sci.agropecu.2025.026Palabras clave:
Phaseolus vulgaris L., catalase, proline, peroxidase, malondialdehyde, seedling vigorResumen
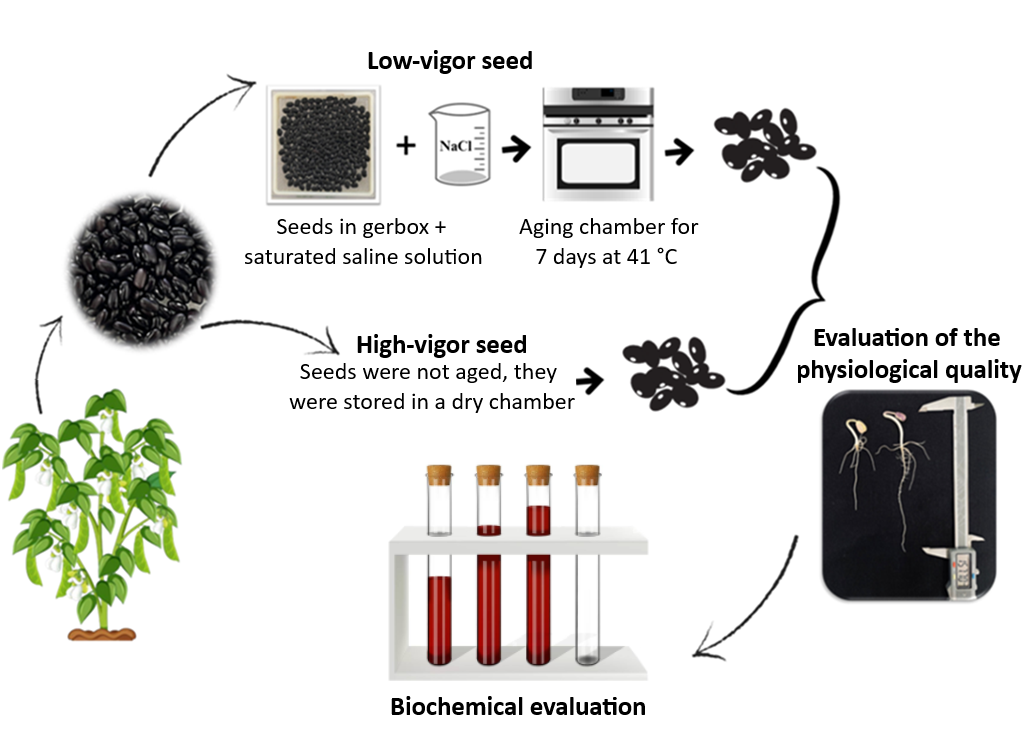
Using seeds with higher physiological potential can help overcome saline stress, affecting many arable areas in tropical and subtropical regions. This study aimed to evaluate whether seed vigor contributes to overcoming saline stress, seeking to identify the association between the antioxidant system and seed lot vigor. Seeds of the BAF55 genotype with two levels of vigor were used. The seeds were germinated under no-stress conditions, with 75 and 150 mmol L-1 of sodium chloride in the solution during germination. After five days, morphological changes and changes in the enzyme’s catalase, ascorbate peroxidase, guaiacol peroxidase, proline, malondialdehyde, and hydrogen peroxide were evaluated. An increase in antioxidant activity was observed with the imposed stresses and no significant difference was observed between the vigor level, except in the condition of 75 mmol L-1 in the hypocotyl of the seedlings and, for proline in the condition of 150 mmol L-1 in which the low-vigor presented greater activity. The stress of 150 mmol L-1 showed greater severity in seeds of low-vigor, resulting in greater lipid peroxidation in the seedlings formed and resulting in seedlings with lower performance.
Citas
Aebi, H. (1984). Catalase in vitro. Methods in enzymology, 105, 121-126. https://doi.org/10.1016/s0076-6879(84)05016-3
Alexieva, V., Sergiev, I., Mapelli, S., & Karanov, E. (2001) The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant, Cell & Environment, 24, 1337-1344. https://doi.org/10.1046/j.1365-3040.2001.00778.x
Alharbi, K., Al-Osaimi, A. A., & Alghamdi, B. A. (2022) Sodium Chloride (NaCl)-Induced Physiological Alteration and Oxidative Stress Generation in Pisum sativum (L.): A Toxicity Assessment. ACS Omega, 7(24), 20819–20832. https://doi.org/10.1021/acsomega.2c01427
Alzahrani, S. M., Alaraidh, I. A., Migdadi, H., Alghamdi, S., Khan, M. A., & Ahmad, P. (2019) Physiological, biochemical, and antioxidant properties of two genotypes of Vicia faba grown under salinity stress. Pakistan Journal of Botany, 51, 786-798. https://doi.org/10.30848/PJB2019-3(3)
Bates, L. S., Waldren, R. P., & Teare, I. D. (1973) Rapid determination of free proline for water-stress studies. Plant and soil, 39, 205-207.
Bewley, J. D., Bradford, K. J., Hilhorst, H. W. M., & Nonogaki, H. (2013) Seeds: physiology of development, germination and dormancy. 3. ed. New York: Springer, 392 p.
Chen, L., Liu, L., Lu, B., Ma, T., Jiang, D., Li, J., Zhang, K., Sun, H., Zhang, Y., Bai, Z., & Li, C. (2020) Exogenous melatonin promotes seed germination and osmotic regulation under salt stress in cotton (Gossypium hirsutum L.). PLoS One, 15, e0228241. https://doi.org/10.1371/journal.pone.0228241
Cheng, X., Xiong, F., Wang, C., Xie, H., He, S., Geng, G., & Zhou, Y. (2018) Seed reserve utilization and hydrolytic enzyme activities in germinating seeds of sweet corn. Pakistan Journal of Botany, 50, 111-116.
Ebone, L. A., Caverzan, A., & Chavarria, G. (2019) Physiologic alterations in orthodox seeds due to deterioration processes. Plant Physiology and Biochemistry, 145, 34-42. https://doi.org/10.1016/j.plaphy.2019.10.028
Egea, I., Estrada, Y., Faura, C., Egea-Fernández, J. M., Bolarin, M. C., & Flores, F. B. (2023) Salt-tolerant alternative crops as sources of quality food to mitigate the negative impact of salinity on agricultural production. Frontiers Plant Science, 14, 1092885. https://doi.org/10.3389/fpls.2023.1092885
Ferreira, D. F. (2011) Sisvar: a computer statistical analysis system. Ciência e agrotecnologia, 35, 1039-1042. https://doi.org/10.1590/S1413-70542011000600001
Hodges, D. M., Delong, J. M., Forney, C. F., & Prange, R. K. (1999) Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta, 207, 604-611. https://doi.org/10.1007/s004250050524
Jianhua, Z., & McDonald, M. B. (1997) The saturated salt accelerated aging test for small-seeded crops. Seed Science and Technology, 25, 123-131.
Kakar, N., Jumaa, S. H., Redoña, E. D., Warburton, M. L., & Reddy, K. R. (2019) Evaluating rice for salinity using pot-culture provides a systematic tolerance assessment at the seedling stage. Rice, 12, 57. https://doi.org/10.1186/s12284-019-0317-7
Marcos-Filho, J. (2015) Seed vigor testing: an overview of the past, present and future perspective. Scientia Agricola, 72, 363-374. https://doi.org/10.1590/0103-9016-2015-0007
McCready, R. M., Guggolz, J., Silviera, V., & Owens, H. S. (1950) Determination of starch and amylose in vegetables. Analytical Chemistry, 22, 1156-1158. https://doi.org/10.1021/ac60045a016
Miller, G. L. (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Analytical chemistry, 31, 426-428. https://doi.org/10.1021/ac60147a030
Mittler, R. (2017) ROS are good. Trends in plant science, 22, 11-19. https://doi.org/10.1016/j.tplants.2016.08.002 1
Monerri, C., & Guardiola, J. L. (1986) Estudio electroforético de las amilasas del guisante. Revista Agroquímica y de Tecnologia de Alimentos, 26, 424-434.
Nadeem, M., Li, J., Yahya, M., Wang, M., Ali, A., Cheng, A., Wang, X., & Ma, C. (2019) Grain legumes and fear of salt stress: Focus on mechanisms and management strategies International Journal of Molecular Sciences, 20, 799. https://doi.org/10.3390/ijms20040799
Nakano, Y., & Asada, K. (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant and Cell Physiology, 22, 867-880. https://doi.org/10.1093/oxfordjournals.pcp.a076232
Padilha, M. S., Coelho, C. M. M., & Sommer, Â. S. (2022) Seed vigor, genotype, and proline in common bean seedling formation under drought and saline stress. Revista Ciência Agronômica, 53, e20228350. https://doi.org/10.5935/1806-6690.20220056
Padilha, M. S., Coelho, C. M. M., Siega, Y. P., & Ehrhardt-Brocardo, N. C. M. (2024) Vigor and reserve mobilization of common bean seed during germination under salt stress conditions. Bragantia, 84, e20240079. https://doi.org/10.1590/1678-4499.20240079
Reis, V. U. V., Penido, A. C., Carvalho, E. R., Rocha, D. K., Reis, L. V., & Semolini, P. H. Z. (2022). Vigor of maize seeds and its effects on plant stand establishment, crop development and grain yield. Journal of Seed Science, 44, 1-12. https://doi.org/10.1590/2317-1545V44257527
Rohman, M. M., Ahmed, I., Molla, M. R., Hossain, M. A., & Maniruzzaman, M. (2019) Evaluation of salt tolerant mungbean (Vigna radiata L.) Genotypes on growth through bio-molecular approaches. Bangladesh Journal of Agricultural Research, 44(3), 469-492. https://doi.org/10.3329/bjar.v44i3.43479
Simões, A. D. N., Moreira, S. I., Mosquim, P. R., Ferreira, N. F., & Puschmann, R. (2015) The effects of storage temperature on the quality and phenolic metabolism of whole and minimally processed kale leaves. Acta Scientiarum Agronomy, 37, 101-107. https://doi.org/10.4025/actasciagron.v37i1.18123
Soares, C., Carvalho, M. E. A., Azevedo, R. A., & Fidalgo, F. (2019) Plants facing oxidative challenges—A little help from the antioxidant networks. Environmental and Experimental Botany, 161, 4-25. https://doi.org/10.1016/j.envexpbot.2018.12.009
Soltani, A., Gholipoor, M., & Zeinali, E. (2006) Seed reserve utilization and seedling growth of wheat as affected by drought and salinity. Environmental and Experimental Botany, 55, 195-200. https://doi.org/10.1016/j.envexpbot.2004.10.012
Taïbi, K., Abderrahim, L. A., Boussaid, M., Bissoli, G., Taïbi, F., Achir, M., Souana, K., & Mulet, J. M. (2021) Salt-tolerance of Phaseolus vulgaris L. is a function of the potentiation extent of antioxidant enzymes and the expression profiles of polyamine encoding genes. South African Journal of Botany, 140, 114-122, 2021. https://doi.org/10.1016/j.sajb.2021.03.045
Wang, P., Liu,W-C., Han, C., Wang, S., Bai, M-Y., & Song, C-P. (2024). Reactive oxygen species: Multidimensional regulators of plant adaptation to abiotic stress and development. Journal of Integrative Plant Biology, 66, 330–367. https://doi.org/10.1111/jipb.13601
Zulfiqar, F., & Ashraf, M. (2023) Proline Alleviates Abiotic Stress Induced Oxidative Stress in Plants. Journal of Plant Growth Regulation, 42, 4629–4651. https://doi.org/10.1007/s00344-022-10839-3
Descargas
Publicado
Cómo citar
Número
Sección
Licencia
Derechos de autor 2025 Scientia Agropecuaria

Esta obra está bajo una licencia internacional Creative Commons Atribución-NoComercial 4.0.
Los autores que publican en esta revista aceptan los siguientes términos:
a. Los autores conservan los derechos de autor y conceden a la revista el derecho publicación, simultáneamente licenciada bajo una licencia de Creative Commons que permite a otros compartir el trabajo, pero citando la publicación inicial en esta revista.
b. Los autores pueden celebrar acuerdos contractuales adicionales separados para la distribución no exclusiva de la versión publicada de la obra de la revista (por ejemplo, publicarla en un repositorio institucional o publicarla en un libro), pero citando la publicación inicial en esta revista.
c. Se permite y anima a los autores a publicar su trabajo en línea (por ejemplo, en repositorios institucionales o en su sitio web) antes y durante el proceso de presentación, ya que puede conducir a intercambios productivos, así como una mayor citación del trabajo publicado (ver efecto del acceso abierto).




