Evaluating light intensity, whey concentration, and pH to enhance biomass and biofuel precursor accumulation in Chlorella vulgaris: A kinetic and experimental approach
DOI:
https://doi.org/10.17268/sci.agropecu.2025.019Palabras clave:
Diseño Box-Benhken, biomasa, recursos energéticos, lípidos, carbohidratos, modelación cinéticaResumen
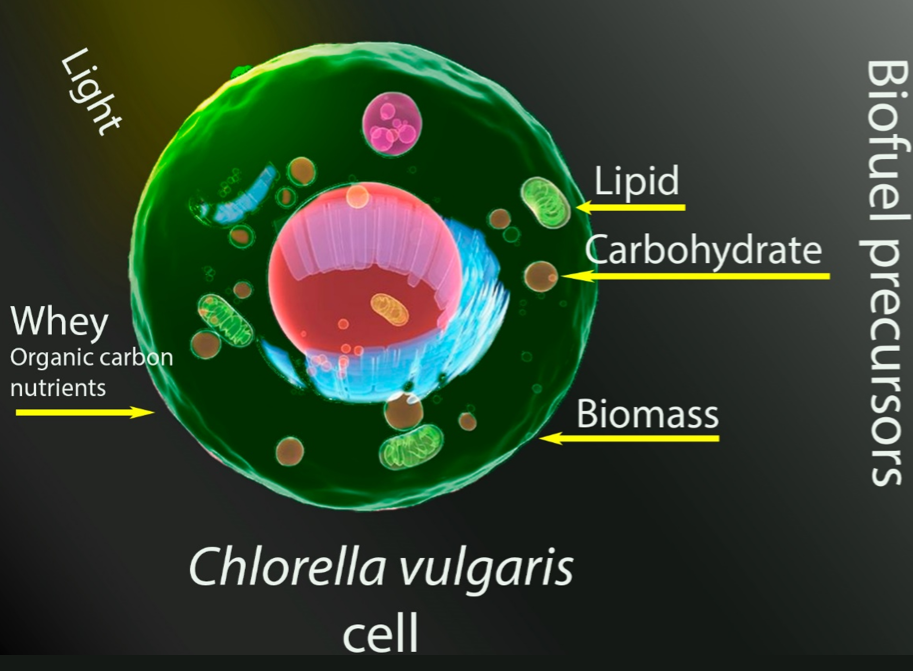
Finding alternatives to fossil fuels is important to mitigate climate change. This study investigates the development of Chlorella vulgaris under different levels of light intensities, whey, and pH from a Box-Behnken experimental design. Maximum biomass growth and lipids/carbohydrate content were measured as response variables. The aim is to understand how the microalga responds to various environmental conditions to optimize its growth and biofuel precursors accumulation. The modified Gompertz kinetic model was adjusted to experimental data to understand how the studied conditions impact the growth rate. As a response, it reached production of 2.17 gbiomass·L-1 at high level of intensity light, whey, and pH. The experiments indicated that the accumulation of lipids/carbohydrates is directly correlated with light intensity; however, the levels of whey concentration and pH played a different role in the production of these compounds, obtaining 88 mglipid·gbiomass-1 and 14.54 mgcarbohydrate·gbiomass-1. Finally, the results showed that under light stress (continuous lighting, which can affect biomass growth due to the diurnal cycle of light and darkness), a high availability of nutrients, and an adequate pH an antagonistic relationship in the synthesis of lipids and carbohydrates was observed, that is, as the concentration of lipids increases, the concentration of carbohydrates decreases. Indicating that these compounds compete for the same carbon precursor and that the microalga prefers to increase the synthesis of lipids instead of carbohydrates since the energy content of lipids is higher than that of carbohydrates.
Citas
Alazaiza, M. Y. D., He, S., Su, D., Abu Amr, S. S., Toh, P. Y., & Bashir, M. J. K. (2023). Sewage water treatment using Chlorella vulgaris microalgae for simultaneous nutrient separation and biomass production. Separations, 10(4), 229. https://doi.org/10.3390/separations10040229
Alharbi, R. M. (2024). Anaerobic co-digestion of cow manure and microalgae to increase biogas production: A sustainable bioenergy source. Journal of King Saud University - Science, 36(9), 103380. https://doi.org/10.1016/j.jksus.2024.103380
Amaral, E. T., Bender, L. B. Y. C., Rizzetti, T. M., & Schneider, R. D. C. D. S. (2023). Removal of organic contaminants in water bodies or wastewater by microalgae of the genus Chlorella: A review. Case Studies in Chemical and Environmental Engineering, 8, 100476. https://doi.org/10.1016/j.cscee.2023.100476
Andrade, M. R., & Costa, J. A. V. (2007). Mixotrophic cultivation of microalga Spirulina platensis using molasses as organic substrate. Aquaculture, 264(1-4), 130-134. https://doi.org/10.1016/j.aquaculture.2006.11.021
AOAC (1980). Official methods of analysis of the association of official analytical chemists. William Horwitz. Washington, D.C., U.S.A. (1980).
Ararat, M. C., Sanclemente Reyes, O. E., & Vergara Patiño, L. (2020). Efecto de la dosificación de CO2 en la cinética de crecimiento de microalgas Chlorella vulgaris y Scenedesmus obliquuss. Revista de Investigación Agraria y Ambiental, 12(1), 89-100. https://doi.org/10.22490/21456453.3482
Ardila-Álvarez, A. M., López-Matos, Y., Vásquez-Cáceres, M. E., González-Delgado, Á. D., & Barajas-Solano, A. F. (2017). Obtención de lípidos y carbohidratos a partir de microalgas mediante el diseño de medios de cultivo selectivos. TecnoLógicas, 20(38), 83. https://doi.org/10.22430/22565337.581
Athanasiadou, V., Klontza, E. E., Dimitriou-Christidis, P., Fountoulakis, M., & Lekkas, D. F. (2023). Evaluation of Arthrospira (Spirulina) platensis growth on cheese whey in the context of circular economy. Sustainable Chemistry and Pharmacy, 34, 101173. https://doi.org/10.1016/j.scp.2023.101173
Baune, M.-C., Januschewski, E., Bussa, M., Van De Walle, S., Gifuni, I., Rodrigues, A. M. C., Cardoso, M. H., Van Royen, G., Juadjur, A., Jungbluth, N., Terjung, N., Castellari, M., & Fanari, F. (2025). Innovative vs. classical methods for drying heterotrophic Chlorella vulgaris: Impact on the nutritional properties, safety, sustainability and costs. Algal Research, 86, 103913. https://doi.org/10.1016/j.algal.2025.103913
Bazdar, E., Roshandel, R., Yaghmaei, S., & Mardanpour, M. M. (2018). The effect of different light intensities and light/dark regimes on the performance of photosynthetic microalgae microbial fuel cell. Bioresource Technology, 261, 350-360. https://doi.org/10.1016/j.biortech.2018.04.026
Bibi, F., Yasmin, H., Jamal, A., AL-Harbi, M. S., Ahmad, M., Zafar, M., Ahmad, B., Samra, B. N., Ahmed, A. F., & Ali, M. I. (2021). Deciphering role of technical bioprocess parameters for bioethanol production using microalgae. Saudi Journal of Biological Sciences, 28(12), 7595-7606. https://doi.org/10.1016/j.sjbs.2021.10.011
Bligh, E. G., & Dyer, W. J. (1959). A rapid method of total lipid extraction and purification. Canadian Journal of Biochemistry and Physiology, 37(8), 911-917. https://doi.org/10.1139/o59-099
Cantú, D., Villegas-Rodríguez, S., Gines-Palestino, R. S., Reyes, J., Cruz, J., & Salinas, D. M. (2024). Harnessing of whey and CO2 for the production of Arthrospira (Spirulina) platensis microalgae biomass: A circular economy approach. Acta Scientiarum. Biological Sciences, 46.
Chojnacka, K., & Noworyta, A. (2004). Evaluation of Spirulina sp. Growth in photoautotrophic, heterotrophic and mixotrophic cultures. Enzyme and Microbial Technology, 34(5), 461-465. https://doi.org/10.1016/j.enzmictec.2003.12.002
Coronado-Reyes, J. A., Salazar-Torres, J. A., Juárez-Campos, B., & González-Hernández, J. C. (2022). Chlorella vulgaris, a microalgae important to be used in Biotechnology: A review. Food Science and Technology, 42, e37320. https://doi.org/10.1590/fst.37320
Daneshvar, E., Antikainen, L., Koutra, E., Kornaros, M., & Bhatnagar, A. (2018). Investigation on the feasibility of Chlorella vulgaris cultivation in a mixture of pulp and aquaculture effluents: Treatment of wastewater and lipid extraction. Bioresource Technology, 255, 104-110. https://doi.org/10.1016/j.biortech.2018.01.101
De Almeida, T., Cardoso, V. L., & Batista, F. R. X. (2022). Feasibility of Chlorella vulgaris to waste products removal from cheese whey. International Journal of Environmental Science and Technology, 19(6), 4713-4722. https://doi.org/10.1007/s13762-021-03423-x
De Carvalho, M. A., Severo Gonçalves, I., Patrícia Held Azambuja, S., Silva Costa, S., Garcia Pereira Silva, P., Oliveira Santos, L., & Goldbeck, R. (2022). Microalgae-based carbohydrates: A green innovative source of bioenergy. Bioresource Technology, 344, 126304. https://doi.org/10.1016/j.biortech.2021.126304
De Farias Silva, C. E., Meneghello, D., De Souza Abud, A. K., & Bertucco, A. (2020). Pretreatment of microalgal biomass to improve the enzymatic hydrolysis of carbohydrates by ultrasonication: Yield vs energy consumption. Journal of King Saud University - Science, 32(1), 606-613. https://doi.org/10.1016/j.jksus.2018.09.007
Debnath, C., Bandyopadhyay, T. K., Bhunia, B., Mishra, U., Narayanasamy, S., & Muthuraj, M. (2021). Microalgae: Sustainable resource of carbohydrates in third-generation biofuel production. Renewable and Sustainable Energy Reviews, 150, 111464. https://doi.org/10.1016/j.rser.2021.111464
Dubois, M., Gilles, K. A., Hamilton, J. K., Rebers, P. A., & Smith, F. (1956). Colorimetric method for determination of sugars and related substances. Analytical Chemistry, 28(3), 350-356. https://doi.org/10.1021/ac60111a017
Ekeuku, S. O., Zainul Azlan, N., Mohd Yusof, Y. A., Tan, J. K., & Makpol, S. (2024). Unveiling the impact of Chlorella vulgaris supplementation on liver metabolisms of aged rats − A preclinical study. Journal of Functional Foods, 121, 106383. https://doi.org/10.1016/j.jff.2024.106383
Esteves, A. F., Salgado, E. M., Vilar, V. J. P., Gonçalves, A. L., & Pires, J. C. M. (2024). A growth phase analysis on the influence of light intensity on microalgal stress and potential biofuel production. Energy Conversion and Management, 311, 118511. https://doi.org/10.1016/j.enconman.2024.118511
Faruque, M. O., Hossain, M. M., & Razzak, S. A. (2025). A green approach to adaptive cultivation of Chlorella sorokiniana in oilfield hypersaline wastewater for sustainable biomass production, nutrient removal, and bioenergy potential. Green Technologies and Sustainability, 3(3), 100179. https://doi.org/10.1016/j.grets.2025.100179
Forero-Cujiño, M. A., Montengro Ruiz, L. C., Pinilla-Agudelo, G. A., & Melgarejo-Muñoz, L. M. (2016). Inmovilización de las microalgas Scenedesmus ovalternus (Scenedesmaceae) y Chlorella vulgaris (Chlorellaceae) en esferas de alginato de calcio. Acta Biológica Colombiana, 21(2), 437-442. https://doi.org/10.15446/abc.v21n2.51253
George, B., Pancha, I., Desai, C., Chokshi, K., Paliwal, C., Ghosh, T., & Mishra, S. (2014). Effects of different media composition, light intensity and photoperiod on morphology and physiology of freshwater microalgae Ankistrodesmus falcatus – A potential strain for bio-fuel production. Bioresource Technology, 171, 367-374. https://doi.org/10.1016/j.biortech.2014.08.086
He, Q., Yang, H., Wu, L., & Hu, C. (2015). Effect of light intensity on physiological changes, carbon allocation and neutral lipid accumulation in oleaginous microalgae. Bioresource Technology, 191, 219-228. https://doi.org/10.1016/j.biortech.2015.05.021
Jiao, H., Tsigkou, K., Elsamahy, T., Pispas, K., Sun, J., Manthos, G., Schagerl, M., Sventzouri, E., Al-Tohamy, R., Kornaros, M., & Ali, S. S. (2024). Recent advances in sustainable hydrogen production from microalgae: Mechanisms, challenges, and future perspectives. Ecotoxicology and Environmental Safety, 270, 115908. https://doi.org/10.1016/j.ecoenv.2023.115908
Josephine, A., Kumar, T. S., Surendran, B., Rajakumar, S., Kirubagaran, R., & Dharani, G. (2022). Evaluating the effect of various environmental factors on the growth of the marine microalgae, Chlorella vulgaris. Frontiers in Marine Science, 9, 954622. https://doi.org/10.3389/fmars.2022.954622
Khalil, Z. I., Asker, M. M. S., El-Sayed, S., & Kobbia, I. A. (2010). Effect of pH on growth and biochemical responses of Dunaliella bardawil and Chlorella ellipsoidea. World Journal of Microbiology and Biotechnology, 26(7), 1225-1231. https://doi.org/10.1007/s11274-009-0292-z
Koochi, Z. H., Jahromi, K. G., Kavoosi, G., & Ramezanian, A. (2023). Fortification of Chlorella vulgaris with citrus peel amino acid for improvement biomass and protein quality. Biotechnology Reports, 39, e00806. https://doi.org/10.1016/j.btre.2023.e00806
Li, D., Yuan, Y., Cheng, D., & Zhao, Q. (2019). Effect of light quality on growth rate, carbohydrate accumulation, fatty acid profile and lutein biosynthesis of Chlorella sp. AE10. Bioresource Technology, 291, 121783. https://doi.org/10.1016/j.biortech.2019.121783
Ma, X., & Jian, W. (2023). Growth conditions and growth kinetics of Chlorella vulgaris cultured in domestic sewage. Sustainability, 15(3), 2162. https://doi.org/10.3390/su15032162
Ma’mun, S., Prasetio, M. W., Anugrah, A. R., Ruliandi, A. P., & Pramuwardani, D. (2024). Bioethanol from Arthrospira platensis biomass using a combined pretreatment. Chemical Engineering Journal Advances, 19, 100616. https://doi.org/10.1016/j.ceja.2024.100616
Martínez Macias, M. D. R., Sánchez Duarte, R. G., Meza Escalante, E. R., Ulloa Mercado, R. G., & Saldívar Cabrales, J. (2017). Síntesis de lípidos de la microalga Nannochloropsis oculata para su uso potencial en la producción de biodiésel. Revista Internacional de Contaminación Ambiental, 33(esp02), 85-91. https://doi.org/10.20937/RICA.2017.33.esp02.08
Mehariya, S., Plöhn, M., Jablonski, P., Stagge, S., Jönsson, L. J., & Funk, C. (2023). Biopolymer production from biomass produced by Nordic microalgae grown in wastewater. Bioresource Technology, 376, 128901. https://doi.org/10.1016/j.biortech.2023.128901
Melo, R. G. D., Andrade, A. F. D., Bezerra, R. P., Correia, D. S., Souza, V. C. D., Brasileiro-Vidal, A. C., Viana Marques, D. D. A., & Porto, A. L. F. (2018). Chlorella vulgaris mixotrophic growth enhanced biomass productivity and reduced toxicity from agro-industrial by-products. Chemosphere, 204, 344-350. https://doi.org/10.1016/j.chemosphere.2018.04.039
Monjed, M. K., Achour, B., Robson, G. D., & Pittman, J. K. (2021). Improved saccharification of Chlorella vulgaris biomass by fungal secreted enzymes for bioethanol production. Algal Research, 58, 102402. https://doi.org/10.1016/j.algal.2021.102402
Montalvo, D., Corro, G., Bañuelos, F., Olivares-Xometl, O., Arellanes, P., & Pal, U. (2023). Selective alcohols production through CO2 photoreduction using Co3O4 /TiO2 photocatalyst exploiting synergetic interactions between Ti3+, Co2+ and Co3+. Applied Catalysis B: Environmental, 330, 122652. https://doi.org/10.1016/j.apcatb.2023.122652
Montalvo-Salinas, D., & Cantú-Lozano, D. (2018). Rheological performance of lactic acid production from whey using Kluyveromyces marxianus yeast. Effect of initial concentrations of substrate, inoculum and oxygen. Ciencia e Ingeniería, 39(3), 215-228.
Montalvo-Salinas, D., Ruiiz-Terán, F., Luna-Solano, G., & Cantú-Lozano, D. (2018). Modeling rheological of whey on function of shear rate, temperature and total solids concentration. Revista Tecnica De La Facultad De Ingenieria Universidad Del Zulia, 41(3), 156-164.
Najar-Almanzor, C. E., Velasco-Iglesias, K. D., Solis-Bañuelos, M., González-Díaz, R. L., Guerrero-Higareda, S., Fuentes-Carrasco, O. J., García-Cayuela, T., & Carrillo-Nieves, D. (2024). Chlorella vulgaris-mediated bioremediation of food and beverage wastewater from industries in Mexico: Results and perspectives towards sustainability and circular economy. Science of The Total Environment, 940, 173753. https://doi.org/10.1016/j.scitotenv.2024.173753
Nguyen, T. D. P., Nguyen, D. H., Lim, J. W., Chang, C.-K., Leong, H. Y., Tran, T. N. T., Vu, T. B. H., Nguyen, T. T. C., & Show, P. L. (2019). Investigation of the relationship between bacteria growth and lipid production cultivating of microalgae Chlorella Vulgaris in seafood wastewater. Energies, 12(12), 2282. https://doi.org/10.3390/en12122282
Nzayisenga, J. C., Farge, X., Groll, S. L., & Sellstedt, A. (2020). Effects of light intensity on growth and lipid production in microalgae grown in wastewater. Biotechnology for Biofuels, 13(1), 4. https://doi.org/10.1186/s13068-019-1646-x
Oliva, G., Buonerba, A., Grassi, A., Hasan, S. W., Korshin, G. V., Zorpas, A. A., Belgiorno, V., Naddeo, V., & Zarra, T. (2024). Microalgae to biodiesel: A novel green conversion method for high-quality lipids recovery and in-situ transesterification to fatty acid methyl esters. Journal of Environmental Management, 357, 120830. https://doi.org/10.1016/j.jenvman.2024.120830
Ozcelik, D., Suwal, S., Ray, C., Tiwari, B. K., Jensen, P. E., & Poojary, M. M. (2024). Valorization of dairy side-streams for the cultivation of microalgae for value added food products. Trends in Food Science & Technology, 146, 104386. https://doi.org/10.1016/j.tifs.2024.104386
Rajivgandhi, G., Ramachandran, G., Chelliah, C. K., Maruthupandy, M., Quero, F., S, V., AL-Mekhlafi, F. A., Wadaan, M. A., Ranjitha, J., & Li, W.-J. (2022). Green microalgal strain Chlorella vulgaris isolated from industrial wastewater with remediation capacity. Environmental Technology & Innovation, 28, 102597. https://doi.org/10.1016/j.eti.2022.102597
Rautenberger, R., Détain, A., Skjånes, K., Schulze, P. S. C., Kiron, V., & Morales-Sánchez, D. (2024). Growth strategies of Chlorella vulgaris in seawater for a high production of biomass and lipids suitable for biodiesel. Algal Research, 77, 103360. https://doi.org/10.1016/j.algal.2023.103360
Rodríguez-González, D., Colominas-Aspuro, A. M., & Zumbado-Fernández, H. M. (2024). Utilización de suero lácteo en la elaboración de una bebida refrescante con fructooligosacáridos y pulpa de acerola (Malpighia emarginata D.C.). Ciencia y Tecnología Agropecuaria, 25(3). https://doi.org/10.21930/rcta.vol25_num3_art:3692
Samiee-Zafarghandi, R., Karimi-Sabet, J., Abdoli, M. A., & Karbassi, A. (2018). Increasing microalgal carbohydrate content for hydrothermal gasification purposes. Renewable Energy, 116, 710-719. https://doi.org/10.1016/j.renene.2017.10.020
Sanjurjo, C., Oulego, P., Bartolomé, M., Rodríguez, E., Gonzalez, R., & Hernández Battez, A. (2024). Biodiesel production from the microalgae Nannochloropsis gaditana: Optimization of the transesterification reaction and physicochemical characterization. Biomass and Bioenergy, 185, 107240. https://doi.org/10.1016/j.biombioe.2024.107240
Silvello, M. A. D. C., Gasparotto, G. A., & Goldbeck, R. (2023). Enzymatic hydrolysis of carbohydrate-rich Chlorella vulgaris for third-generation bioethanol production by cellulase-recombinant yeast. Cleaner Chemical Engineering, 6, 100111. https://doi.org/10.1016/j.clce.2023.100111
Soleimani Khorramdashti, M., Samipoor Giri, M., & Majidian, N. (2021). Extraction lipids from Chlorella vulgaris by supercritical CO2 for biodiesel production. South African Journal of Chemical Engineering, 38, 121-131. https://doi.org/10.1016/j.sajce.2021.03.008
Van Nerom, S., Buyse, K., Van Immerseel, F., Robbens, J., & Delezie, E. (2024). Pulsed electric field (PEF) processing of microalga Chlorella vulgaris and its digestibility in broiler feed. Poultry Science, 103(6), 103721. https://doi.org/10.1016/j.psj.2024.103721
Vyas, S., Patel, A., Nabil Risse, E., Krikigianni, E., Rova, U., Christakopoulos, P., & Matsakas, L. (2022). Biosynthesis of microalgal lipids, proteins, lutein, and carbohydrates using fish farming wastewater and forest biomass under photoautotrophic and heterotrophic cultivation. Bioresource Technology, 359, 127494. https://doi.org/10.1016/j.biortech.2022.127494
Wong, Y. K., Ho, Y. H., Ho, K. C., Leung, H. M., & Yung, K. K. L. (2017). Maximization of cell growth and lipid production of freshwater microalga Chlorella vulgaris by enrichment technique for biodiesel production. Environmental Science and Pollution Research, 24(10), 9089-9101. https://doi.org/10.1007/s11356-016-7792-9
Yu, H., Chen, X., Du, X., Chang, Y., Sun, S., Tang, S., Du, Q., & Song, W. (2024). Exploring the molecular mechanism of Chlorella vulgaris in response to androstenedione exposure based on genes continuously up-regulated in transcription analysis. Ecotoxicology and Environmental Safety, 271, 115996. https://doi.org/10.1016/j.ecoenv.2024.115996
Zhang, K., Sun, B., She, X., Zhao, F., Cao, Y., Ren, D., & Lu, J. (2014). Lipid production and composition of fatty acids in Chlorella vulgaris cultured using different methods: Photoautotrophic, heterotrophic, and pure and mixed conditions. Annals of Microbiology, 64(3), 1239-1246. https://doi.org/10.1007/s13213-013-0766-y
Descargas
Archivos adicionales
Publicado
Cómo citar
Número
Sección
Licencia
Derechos de autor 2025 Scientia Agropecuaria

Esta obra está bajo una licencia internacional Creative Commons Atribución-NoComercial 4.0.
Los autores que publican en esta revista aceptan los siguientes términos:
a. Los autores conservan los derechos de autor y conceden a la revista el derecho publicación, simultáneamente licenciada bajo una licencia de Creative Commons que permite a otros compartir el trabajo, pero citando la publicación inicial en esta revista.
b. Los autores pueden celebrar acuerdos contractuales adicionales separados para la distribución no exclusiva de la versión publicada de la obra de la revista (por ejemplo, publicarla en un repositorio institucional o publicarla en un libro), pero citando la publicación inicial en esta revista.
c. Se permite y anima a los autores a publicar su trabajo en línea (por ejemplo, en repositorios institucionales o en su sitio web) antes y durante el proceso de presentación, ya que puede conducir a intercambios productivos, así como una mayor citación del trabajo publicado (ver efecto del acceso abierto).




