Effect of moderate heat stress on tuberization of potato genotypes: Morphological and physiological changes using in vitro and ex vitro propagation
DOI:
https://doi.org/10.17268/sci.agropecu.2024.010Palabras clave:
Canchán, chlorocholine chloride, heat tolerance, microtubers, Solanum tuberosumResumen
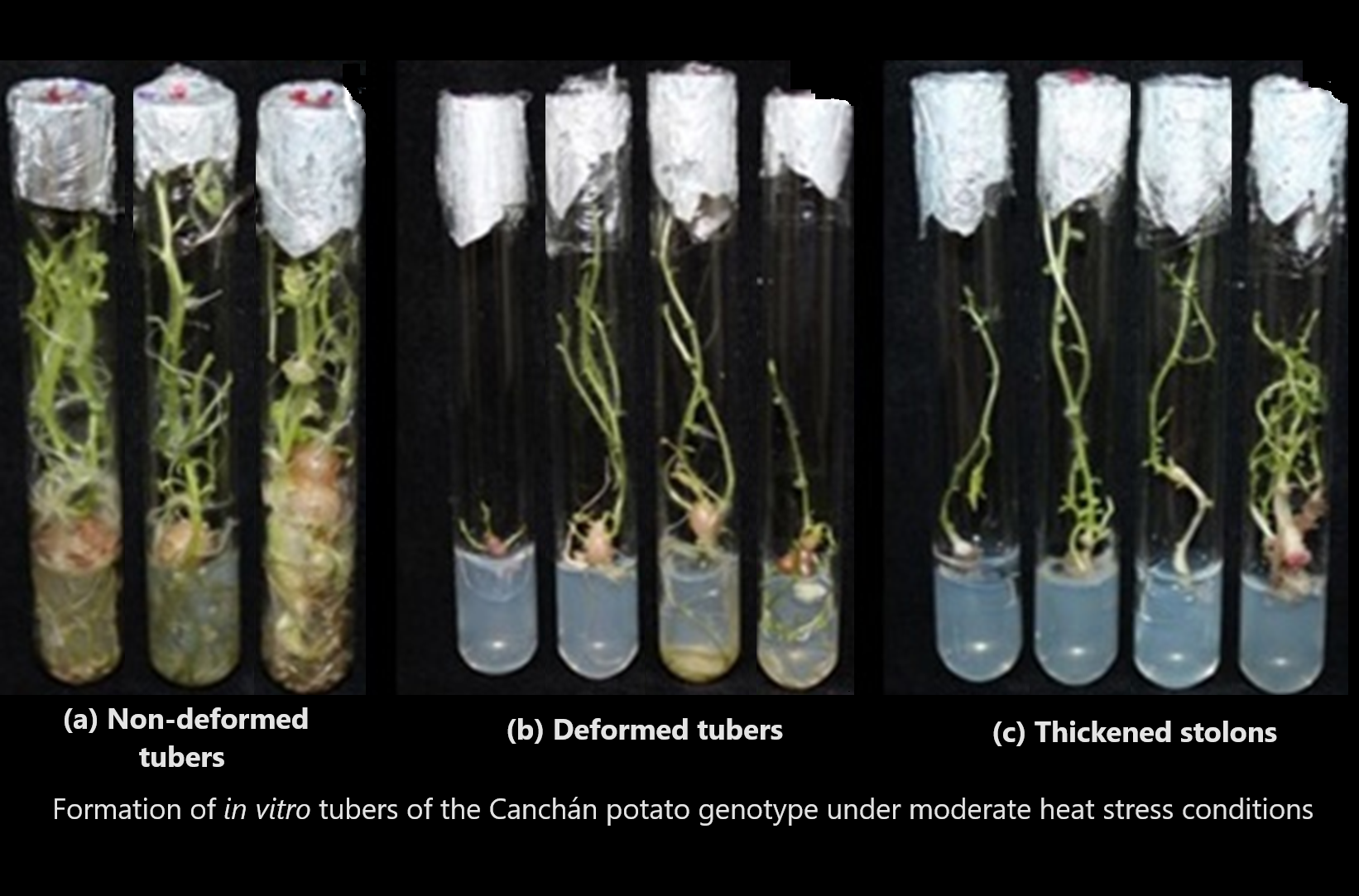
Potato plants are often subjected to heat stress during growth and development, resulting in loss of yield and quality. However, the possibility exists that commercially important genotypes show tolerance to heat stress. In this study, we aim to investigate the morphological and physiological effects of moderate conditions of heat stress on tuberization in nine potato genotypes, and how it affects propagation using an ex vitro and in vitro system. The plant material came from the In Vitro Germplasm Bank of the International Potato Center (CIP). The incubation temperatures of the cultures in in vitro and ex vitro conditions were the ambient in the laboratory and greenhouse, respectively. In in vitro system a randomized complete block design was employed in nine genotypes, two treatments: multiplication culture medium and the same culture medium supplemented with 8.0% sucrose and 500 mg/L-1 CCC, six flasks and five explants per experimental unit. Among all genotypes evaluated, Leona and Liberteña did not form tubers under ex vitro treatment conditions. Under in vitro conditions, Amarilis, Capiro, Perricholi and Tacna showed no evidence of tuberization, and Canchán was the only genotype where 100% formation of tuberous structures were produced in all evaluations. The findings concluded in this study showed that of all the potatoes evaluated, Canchán best responded to the evaluations under both in ex vitro and in vitro conditions, at maximum temperatures between 22.4 to 26.9 and 27.0 to 28.2 oC, respectively. Therefore, it is vitally important to consider its development potential in different producing locations around the world, which are directly and indirectly affected by climate change.
Citas
Ahloowalia, B. S. (1994). Production and performance of potato mini-tubers. Euphytica 75, 163-172.
Basu, P. S., & Minhas, J. S. (1991). Heat tolerance and assimilate transport in different potato genotypes. Journal of Experimental Botany 42(7), 861-866.
Birch, P. R., Bryan, G., Fenton, B., Gilroy, E. M., Hein, I., et al. (2012). Crops that feed the world 8: Potato: Are the trends of increased global production sustainable? Food Security 4, 477-508.
Campbell, R., Ducreux, L., Cowan, G., Young, V., Chinoko, G., et al. (2022). Allelic variants of a potato HEAT SHOCK COGNATE 70 gene confer improved tuber yield under a wide range of environmental conditions. Food and Energy Security 12(1): e377.
Charfeddini, M., Chiab, N., Charfeddini, S., Ferjani, A., Gargouri-Bouzid, R. (2023). Heat, drought, and combined stress effect on transgenic potato plants overexpressing the StERF94 transcription factor. Journal of Plant Research 136(4), 549-562.
Chaudhary, S., Devi, P., Bhardwaj, A., Jha, U. C., Sharma, K. D., et al. (2020). Identification and characterization of contrasting genotypes/cultivars for developing heat tolerance in agricultural crops: current status and prospects. Frontiers in Plant Science 2020, 11,587264.
Chen, C.-T., & Setter, T. L. (2021). Role of tuber developmental processes in response of potato to high temperature and elevated CO2. Plants 2021, 10:871.
Dalimunthe, R. H., Setiado, H., Lubis, K., & Damanik, R. I. 2021. Effect of paclobutrazol in micro tuberization of potato (Solanum tuberosum L.) cultivar Granola Kembang and Repita. IOP Conference Series. Earth and Environmental Science 782(4), 042058.
Estrada, R., Tovar, P., & Dodds, J. H. (1986). Induction of in vitro tubers in a broad range of genotypes. Plant Cell Tissue and Organ Culture 7(1), 3-10.
Fedorova, J. N., Lebedeva, N. V., & Fedorova, L. N. (2021). The adaptation of in vitro potato materials applying microbiologic substances. In: Bogoviz AV (eds). The Challenges of Sustainability in Agricultural Systems. Lectures Notes in Networks and Systems 206, pp. 761-770.
Fufa, M., & Diro, M. (2014) Microtuber induction of two potato (Solanum tuberosum L.) varieties. Advances in Crop Science and Technology 2, 122.
Garner, N., & Blake, J. (1989). The induction and development of potato microtubers in vitro on media free of growth regulating substances. Annals of Botany 63, 663-674.
Gautam, S., Solis-García, N., Teale, M. K., Mandadi, K., da Silva, J. A., et al. (2021). Development of an in vitro microtuberization and temporary immersion bioreactor system to evaluate heat stress tolerance in potatoes (Solanum tuberosum L.). Frontiers in Plant Science 12, 700328.
Gelmesa, D., Dechassa, N., Mohammed, W., Gebre, E., Monneveux, P., et al. (2017). In vitro screening of potato genotypes for osmotic stress tolerance. Open Agriculture 2(1), 308-316.
Gómez-Zavaglia, A., Mejuto, J. C., & Simal-Gandara, J. 2020. Mitigation of emerging implications of climate change of food production systems. Food Research International 134, 109256.
Gopal, J., Minocha, J., & Dhaliwal. H. (1998). Microtuberization in potato (Solanum tuberosum L.). Plant Cell Reports 17(10), 794-98.
Gull, A., Lone, A. A., & Wani, N. U. I. (2019). Biotic and abiotic stresses in plants. In: De Oliveira AB (ed.). Abiotic and Biotic Stress in Plants. IntechOpen 2019.
Hancock, R. D., Morris, W. L., Ducreux, L. J. M. M., Morris, J. A., Usman, M., et al., (2014). Physiological, biochemical and molecular responses of the potato (Solanum tuberosum L.) plant to moderately elevated temperature. Plant Cell & Environment 37(2), 439-50.
Harun-Or-Rashid, M., Islam, S. M. S., Miah, M. A. B., Subramaniam, S. (2024). Evaluation of the thermo-tolerance effect on cell suspension culture in potato (Solanum tuberosum L.). Potato Research. https://doi.org/10.1007/s11540-024-09692-6
Herrera-Isidron, L., Valencia-Lozano, E., Rosiles-Loeza, P. Y., Robles-Hernández, M. G., Napsuciale-Heredia, A., et al. (2021). Gene expression analysis of microtubers of potato Solanum tuberosum L. induced in cytokinin containing medium and osmotic stress. Plants. 10(5), 876.
INIA. Papa INIA 303 – Canchán. (2012). Instituto Nacional de Innovación Agraria – INIA. Ministerio de Agricultura. Plegable No 2. 2 p. Lima, Perú.
Kaushal, N., Bhandari, K., Siddique, K. H. M., & Nayyar, H. (2016). Food crops face rising temperatures: an overview of responses, adaptive mechanisms, and approaches to improve heat tolerance. Cogent Food and Agriculture 2, 1134380.
Kim, J.-U. & Lee, B.-W. (2019). Differential mechanisms of potato yield los induced by high day and night temperatures during tuber initiation and bulking: Photosynthesis and tuber growth. Frontiers in Plant Sciences 2019, 10:300.
Kolachevskaya, O. O., Myakushina, Y. A., Getman, I. A., Lomin, S. N., Deyneko, I. V., et al. (2021). Hormonal regulation and crosstalk of auxin/cytokinin signaling pathways in potatoes in vitro and in relation to vegetation or tuberization stages. International Journal of Molecular Science 22(15), 8207.
Lal, M. K., Tiwari, R. K., Kumar, A., Dey, A., Kumar, R., et al. (2022). Mechanistic concept of physiological, biochemical, and molecular responses of the potato crop to heat and drought stress. Plants 11, 2857.
Li, B., Gao, K., Ren, H., & Tang, W. (2018). Molecular mechanisms governing plant responses to high temperatures. Journal of Integrative Plant Biology 60(9), 757-79.
López-Delgado, H., & Scott, I. M. (1997). Induction of in vitro tuberization of potato microplants by acetylsalicylic acid. Journal of Plant Physiology 151(1), 74-78.
Mamiya, K., Tanabe, K., & Onishi, N. (2020). Production of potato (Solanum tuberosum L.) microtubers using plastic culture bags. Plant Biotechnology 37, 233-238.
MIDAGRI (Ministerio de Desarrollo Agrario y Riego). (2018). Plan Nacional de Cultivos (Campaña Agrícola 2018-2019). 293 p.
Mohamed, A. E.-S., & Girgis, N. D. (2023). Factors affecting in vitro tuberization of potato. Bulletin of the National Research Center 47, 80.
Murashige, T., & Skoog, F. (1962). A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiologia Plantarum 15(3), 473-497.
Pérez-Clemente, R. M., & Gómez-Cadenas, A. (2012). In vitro tissue culture, a tool for the study and breeding of plants subjected to abiotic stress conditions. In: Leva A, Rinaldi LMR (Eds.). Recent Advances in Plant in vitro Culture. 222 p.
Pundir, R. K., Pathak, A., Upadhyaya, D. C., Muthusamy, A., & Upadhyaya, C. P. 2021. Red and blue light-emitting diodes significantly improve in vitro tuberization of potato (Solanum tuberosum L.). Journal of Horticultural Research 29(1), 95-108.
Rykaczewska, K. (2013). The impact of high temperature during growing season on potato cultivars with different response to environmental stresses. American Journal of Plant Science 4(12), 2386-2393.
Rykaczewska, K. (2015). The effect of high temperature occurring in subsequent stages of plant development on potato yield and tuber physiological defects. American Journal of Potato Research 92(3), 339-349.
Singh, A., Siddappa, S., Bhardwaj, V., Singh, B., Kumar, D., et al. (2015). Expression profiling of potato cultivars with contrasting tuberization at elevated temperature using microarray analysis. Plant Physiology and Biochemistry. 97, 108-116.
Singh, A., Kaushai, N., Sharma, R., Bhardway, V., Singh, B., et al. (2016). Effect of elevated temperature on in vitro microtuberization of potato genotypes with different thermotolerance levels. Vegetos. International Journal of Plant Research 29(3), 6.
Struik, P. C. (2007). Responses of the potato plant to temperature. In: Potato Biology and Biotechnology: Advances and Perspectives. Eds. D. Vreugdenhil, J. Bradshaw, C. Gebhardt, F. Govers, D. K. L. MacKerron, M. A. Taylor, et al. (Amsterdam: Elsevier). 2007, 366-396.
Timlin, D., Rahman, S. M. L., Baker, J., Reddy, V. R., Fleisher, D. et al. (2006). Whole plant photosynthesis, development, and carbon partitioning in potato as a function of temperature. Agronomy Journal 98, 1195-1203.
Tiwari, R. K., Lal, M. K., Naga, K. C., Kumar, R., Chourasia, K. N., et al. (2020). Emerging roles of melatonin in mitigating abiotic and biotic stresses of horticultural crops. Scientia Horticulturae 272, 109592.
Tovar, P., Estrada, R., Shilde-Rentschler, L., & Dodds, J. H. (1985). Induction of in vitro potato tubers. CIP Circular 13(4), 1-4. International Potato Center, Lima, Peru.
Waqas, M. A., Kaya, C., Riaz, A., Farooq, M., Nawaz, I., et al. (2019). Potential mechanisms of abiotic stress tolerance in crop plants induced by thiourea. Frontiers in Plant Science 10, 1336.
Zhang, S., Ye, H., Kong, L., Li, X., Chen, Y., et al. (2024). Multivariate analysis compares and evaluates heat tolerance of potato germplasm. Plants 13(1), 142.
Descargas
Publicado
Cómo citar
Número
Sección
Licencia
Derechos de autor 2024 Scientia Agropecuaria

Esta obra está bajo una licencia internacional Creative Commons Atribución-NoComercial 4.0.
Los autores que publican en esta revista aceptan los siguientes términos:
a. Los autores conservan los derechos de autor y conceden a la revista el derecho publicación, simultáneamente licenciada bajo una licencia de Creative Commons que permite a otros compartir el trabajo, pero citando la publicación inicial en esta revista.
b. Los autores pueden celebrar acuerdos contractuales adicionales separados para la distribución no exclusiva de la versión publicada de la obra de la revista (por ejemplo, publicarla en un repositorio institucional o publicarla en un libro), pero citando la publicación inicial en esta revista.
c. Se permite y anima a los autores a publicar su trabajo en línea (por ejemplo, en repositorios institucionales o en su sitio web) antes y durante el proceso de presentación, ya que puede conducir a intercambios productivos, así como una mayor citación del trabajo publicado (ver efecto del acceso abierto).




