UV-C priming enhances antioxidant mechanisms and bioactive compound biosynthesis in broccoli sprouts
M. A. C. Centeno-Rodríguez1 ; J. A. Gómez-Salazar1, 2 ; J. E. Ruiz-Nieto1, 3
M. A. Martínez-Téllez4 ; L. E. Casados-Vázquez1, 2 ; M. E. Sosa-Morales1, 2 ; A. Cerón-García1, 2 *
1 Posgrado en Biociencias, Universidad de Guanajuato. Carretera Irapuato-Silao km 9, 36500, Irapuato, Guanajuato, México.
2 Departamento de Alimentos, División Ciencias de la Vida, Universidad de Guanajuato. Carretera Irapuato-Silao km 9, 36500, Irapuato, Guanajuato, México.
3 Departamento de Agronomía, División Ciencias de la Vida. Universidad de Guanajuato. Carretera Irapuato, Silao km 9, 36500, Irapuato, Guanajuato, México.
4 Centro de Investigación en Alimentación y Desarrollo, A. C. Carretera Gustavo Enrique Astiazarán Rosas, No. 46, Col. La Victoria, 83304, Hermosillo, Sonora, México.
* Corresponding author: abel.ceron@ugto.mx (A. Cerón-García).
Received: 18 February 2025. Accepted: 5 August 2025. Published: 1 September 2025.
Abstract
Foods as a source of bioactive compounds has led to the development of agronomic strategies to enhance their levels. In this study, the effect of UV-C radiation as a pre-germinative treatment on broccoli was evaluated. Key parameters were analyzed, such as stress markers (proline and H2O2), photosynthetic pigments (chlorophylls and carotenoids), total phenolic compounds, total flavonoids, enzymatic antioxidant activity (superoxide dismutase, SOD; catalase, CAT; and ascorbate peroxidase, APX), and biocompound-related enzymes. Additionally, 𝛽-carotene, lutein and phenolic compounds profiles were determined using chromatographic techniques. The results revealed that UV-C pre-treatment induced a significant defense response in broccoli sprouts, including increased proline synthesis, enhanced CAT and APX activity, and accumulation of TF. On the other hand, a reduction of photosynthetic pigments was observed. These biochemical changes occurred without significant phenotypical alterations. In conclusion, UV-C radiation as a pre-germinative treatment enhances the bioactive quality of broccoli sprouts by promoting stress-related metabolic pathways, suggesting its potential as a practical agronomic strategy for improving their functional value.
Keywords: proline; biocompounds; antioxidant system; broccoli sprouts; UV-C radiation.
DOI: https://doi.org/10.17268/sci.agropecu.2025.049
Cite this article:
Centeno-Rodríguez, M. A. C., Gómez-Salazar, J. A., Ruiz-Nieto, J. E., Martínez-Téllez, M. A., Casados-Vásquez, L. E., Sosa-Morales, M. E., Cerón-García, A. (2025). UV-C priming enhances antioxidant mechanisms and bioactive compound biosynthesis in broccoli sprouts. Scientia Agropecuaria, 16(4), 647-657.
1. Introduction
Nowadays, consumers are increasingly aware of the impact that diet has on health, life expectancy, and overall quality of life (Piechowiak & Balawejder, 2025; Abellán et al., 2019). This growing awareness has led to a rising interest in exploring and evaluating new approaches to enhance the functional properties of food (Wang et al., 2023). Among these, edible sprouts, particularly broccoli sprouts, have achieved special attention due to their rich content of micronutrients, including vitamins C and E, minerals, amino acids, and secondary metabolites such as carotenoids, phenolic compounds, and glucosinolates. These compounds have been associated with reduced risks of chronic degenerative diseases linked to oxidative stress (Li et al., 2025; Li et al., 2024; Xia et al., 2024; Ampofo & Ngadi, 2020; Abellán et al., 2019).
In plants, secondary metabolites play a crucial role in defense responses (Paucar-Menacho et al., 2017). Leveraging this characteristic, controlled stress conditions can be applied to stimulate metabolic pathways and physiological processes, inducing defense responses and enhancing the accumulation of health-promoting phytochemicals in plant systems (Ampofo & Ngadi, 2020).
Manipulating sprout production conditions offers an opportunity to increase the production of secondary metabolites both pre- and postharvest (Abellán et al., 2019). Among the factors influencing this process, light stands out as a vital element for plant growth and development. Specifically, ultraviolet (UV) light -a form of electromagnetic radiation within the 100 – 400 nm range- is subdivided into UV-A (315 – 400 nm), UV-B (280 – 315 nm), and UV-C (100 – 280 nm) (Hernández-Aguilar et al., 2021). UV light application has emerged as one of the most effective methods to enhance the content of bioactive compounds in seeds and sprouts (Urban et al., 2016).
The effects of UV radiation on cellular processes and metabolite biosynthesis vary depending on wavelength, dose, and duration of exposure. While UV light can induce abiotic stress signaling in plants, resulting in the production of reactive oxygen species (ROS), excessive ROS levels can cause oxidative stress (Rizi et al., 2021). To mitigate this, plants have evolved protection mechanisms, including the activation of antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT), and ascorbate peroxidase (APX), which neutralize ROS.
For example, Cao et al. (2020) demonstrated that postharvest exposure to UV-C radiation (30 W, 10 min) significantly increased CAT activity in mung bean sprouts. Additionally, UV light exposure promotes the biosynthesis of secondary metabolites and the accumulation of photoprotective bioactive compounds such as anthocyanins and carotenoids (Rizi et al., 2021). Ji et al. (2016) observed that UV-C light exposure (70.32 W/m² for 12 h prior to harvest) significantly enhanced phenylalanine ammonia-lyase (PAL) activity in buckwheat sprouts, leading to increased flavonoid content.
Also, low-molecular-weight compounds, such as proline, play a critical role in plant stress responses, acting as signaling molecules and contributing to antioxidant defense mechanisms (Alagoz et al., 2023). While many studies have examined the effects of UV irradiation, particularly UV-A and UV-B light, which are effective when applied over extended periods, UV-C light holds promise as an efficient inducer of secondary metabolite synthesis due to its higher photon energy (Urban et al., 2016). However, research on the impact of UV-C light on broccoli seeds and sprouts remains limited.
Therefore, the objective of this study was to evaluate the effects of UV-C light on antioxidant enzyme activity, phenolic compound synthesis, and the response of proline, photosynthetic pigments, and phenotypic parameters under controlled UV-C stress conditions of broccoli sprouts.
2. Methodology
2.1 Plant material
Two grams of commercial broccoli seeds (Brassica oleracea, variety Waltham 29) without chemical treatment were washed and disinfected in a 1% NaClO solution for 5 min. Seeds were then rinsed five times with purified water to remove any NaClO residues.
2.2 UV-C radiation treatment and germination conditions
Disinfected seeds (2 g) were placed in duplicate in plastic Petri dishes (60 mm x 15 mm) and subjected to UV-C radiation inside a UV-C light chamber (254 nm, intensity of 10 mW/cm², Crob/4S model lamps, Instrumart, Mexico). The exposure time was 10 min at distance of 8 cm, corresponding to a radiation dose of 60 kJ/m². To ensure uniform exposure, 50% of the dose was applied initially, followed by manual homogenization of the seeds, and then the remaining dose was applied (García-Mosqueda et al. 2023). The radiation dose was calculated using the following equation and expressed in kJ/m².
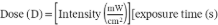 ]
]
After treatment, the seeds were transferred to plastic trays (8 x 3 x 30 cm) and placed in a controlled growth chamber set at 24 °C ± 2 °C with a 16/8 h light/dark photo period for 7 days to allow germination. The sprouts were harvested, with some used for phenotypic characterization and the remainder was freeze-dried (Freeze Dryer FDB-5502, OPERON, Korea) for further analysis. Sprouts grown from untreated seeds were used as controls. All experiments were performed in duplicate.
2.3 Determination of stress markers
2.3.1 Proline content
Proline content was evaluated following the modified method of Bates et al. (1973). A lyophilized sample (50 mg) was homogenized with 3 mL of distilled water. The homogenate was centrifuged at 9,500 x g for 20 min at 4 °C (Centrifuge Hermle Z326, Labortechnik GmbH, Germany), and 111 µL of the supernatant was reacted with 56 µL of 85% formic acid and 222 µL of a freshly prepared 3% ninhydrin solution (using ethylene glycol monomethyl ether as solvent). The mixture was boiled for 15 min, cooled to room temperature for 5 min, and mixed with 1.112 mL of 50% isopropyl alcohol. Absorbance was measured at 520 nm (Spectrophotometer UV/Vis, Perkin Elmer, USA) and results were expressed as micrograms of proline per gram of dry weight (µg/g DW).
2.3.2 Hydrogen peroxide (H₂O₂) content
H₂O₂ content was estimated following the modified method of Ampofo & Ngadi (2020). A lyophilized sample (100 mg) was homogenized in 5 mL of 0.1% trichloroacetic acid (TCA) at 4 °C and centrifuged at 10,000 x g for 15 min at 4 °C (Centrifuge Hermle Z326, Labortechnik GmbH, Germany). A clarified extract (500 µL) was mixed with 500 µL of potassium phosphate buffer (10 mM, pH 7.0) and 1 mL of 1 M potassium iodide. After 30 min of incubation in the dark at 4 °C, absorbance was recorded at 390 nm (Spectrophotometer UV/Vis, Perkin Elmer, USA). For the blank, plant extract was replaced with 0.1% TCA. Results were expressed millimolar concen-tration of H₂O₂ per gram of dry weight (mM H₂O₂/g DW).
2.3.3 Stress indicator enzymes
Total protein assay. Protein contents of enzyme extracts were measured using the BioRad® protein determination reagent (catalog 5000006). A 100 µL aliquot of enzyme extract was mixed with 1 mL of Bradford reagent and incubated for 10 min at room temperature. The absorbance was then measured at 595 nm (Spectrophotometer UV/Vis, Perkin Elmer, USA). For the blank, the enzyme extract was replaced with the extraction buffer. All enzyme assays were performed on the same day as the extraction.
Superoxide Dismutase (SOD). A freeze-dried sample (50 mg) was mixed in a cold mortar with 5% polyvinyl polypyrrolidone (PVPP) and 2 mL of extraction buffer (1 mM ethylene diamine tetracetic acid (EDTA), in 50 mM potassium phosphate buffer, pH 7.5). The mixture was then centrifuged at 12,000 × g for 15 min at 4 °C (Centrifuge Hermle Z326, Labortechnik GmbH, Germany). A 25 µL aliquot of the supernatant, equivalent to 50 µg of protein, was collected and mixed with 1 mL of the reaction mixture (57 µM nitroblue tetrazolium (NBT), 0.025% Triton X-100, 0.11 mM EDTA, 10 mM L-methionine, in 0.1 M sodium phosphate buffer, pH 7.8,) and 44 µL of 60 µM riboflavin. The reaction was initiated by the addition of riboflavin, and the samples were exposed to fluorescent light (30 W, 25 °C) for 30 min. The absorbance was then measured at 560 nm (Spectrophotometer UV/Vis, Perkin Elmer, USA). For the blank, the extraction buffer was added instead of the enzyme extract. One unit of SOD was defined as the amount of enzyme that inhibits 50% of the NBT photoreduction per min. The results were expressed as units per mg of protein (U/mg protein) (Ozuna et al., 2018).
Catalase (CAT). The catalase activity was measured according to Ozuna et al. (2018) method. A freeze-dried sample (100 mg) was homogenized with 10 mL of extraction buffer (50 mM phosphate-K buffer, pH 7.8, 0.05% Triton X-100, 1% PVPP, and 0.5 mM EDTA). The mixture was then centrifuged at 18,000 × g for 20 min at 4 °C (Centrifuge Hermle Z326, Labortechnik GmbH, Germany). An aliquot equivalent to 1 mg of protein (25 µL) was mixed with 470 µL of potassium phosphate buffer (50 mM, pH 7.0) and 5 µL of H2O2. Absorbance was measured immediately at 240 nm at 30 °C in kinetic mode (with a 20-second lag phase and a 180-second reaction period; readings were taken every 60 sec; Spectrophotometer UV/Vis, Perkin Elmer, USA). One unit of CAT was defined as the amount of enzyme that decomposes 1 mmol of H2O2 per min. The results were expressed as U/mg protein.
Ascorbate Peroxidase (APX). For ascorbate peroxidase activity, 100 mg of freeze-dried sprouts was homogenized with 10 mL of extraction buffer (50 mM phosphate-K buffer, pH 7.8, 5 mM ascorbic acid, 1% PVPP, and 0.5 mM EDTA). The mixture was then centrifuged at 18,000 × g for 20 min at 4 °C (Centrifuge Hermle Z326, Labortechnik GmbH, Germany). An aliquot equivalent to 1 mg of protein (50 µL) was mixed with 395 µL of potassium phosphate buffer (50 mM, pH 7.0), 50 µL of 5 mM ascorbate, and 5 µL of H2O2. Absorbance was immediately measured at 290 nm at 25 °C in kinetic mode (with a 20-second lag phase and a 180-second reaction period; readings were taken every 60 sec; Spectrophotometer UV/Vis, Perkin Elmer, USA). One unit of APX was defined as the amount of enzyme that decomposes 1 µmol of H2O2 per min. The results were expressed as U/mg protein (Ozuna et al., 2018).
2.4 Determination of bioactive compounds
2.4.1 Total phenolic compounds
The content of total phenols was determined as described by Slinkard & Singleton (1977). A mortar extract was prepared by mixing 50 mg of lyophilized material with 5 mL of 80% MeOH. The mixture was shaken at 60 rpm for 1 h in the dark. Afterward, the extract was centrifuged at 9,500 × g for 10 min at 4 °C (Centrifuge Hermle Z326, Labortechnik GmbH, Germany). An aliquot of 200 µL of the supernatant was mixed with 200 µL of Folin-Ciocalteu's reagent (diluted in a 1:4 ratio) and 2 mL of 0.5 % sodium carbonate. The mixture was allowed to stand in the dark for 1 h. Finally, absorbance was measured at 760 nm. The results were expressed as milligram gallic acid equivalents per gram of dry weight (mg GAE/g DW).
2.4.2 Total flavonoids
An extract was prepared by mixing 50 mg of lyophilized sample with 5 mL of 80% MeOH. The resulting extract was placed in screw-capped glass tubes and boiled for 1 h with constant stirring. After boiling, the extract was centrifuged at 9,500 × g for 10 min (Centrifuge Hermle Z326, Labortechnik GmbH, Germany). A reaction mixture was then prepared by combining 250 µL of the clarified extract, 50 µL of 10% (w/v) aluminum chloride, 50 µL of 1 M potassium acetate, 800 µL of 80% MeOH, and 1.4 mL of distilled water. Finally, absorbance was measured at 415 nm. The results were expressed as milligram quercetin 3-glucoside equivalents per gram of dry weight (mg EQ 3-glc/g DW) (Khanam et al., 2012).
2.4.3 Precursor enzymes for the synthesis of phenolic compounds
Enzyme extractions: Phenylalanine ammonia-lyase (PAL) and tyrosine ammonia-lyase (TAL) extractions were performed at 4 °C. Briefly, 50 mg of freeze-dried sample was homogenized with 2 mL of extraction buffer (50 mM borate buffer, pH 8.8) for 20 min. The mixture was then centrifuged at 12,000 x g for 30 min at 4 °C (Centrifuge Hermle Z326, Labortechnik GmbH, Germany), and the supernatant was collected as the enzyme extract. Enzyme activity assays were conducted on the same day as extraction (Ampofo & Ngadi, 2020).
Phenylalanine ammonia-lyase assay. For the determination of PAL, a reaction mixture was prepared containing 300 µL of enzyme extract (equivalent to 1 mg of protein), 1.2 mL of 0.02 M L-phenylalanine, and 2 mL of extraction buffer. The mixture was incubated at 30 °C for 1 h. The reaction was stopped by adding 500 µL of 10% TCA, followed by centrifugation at 15,000 x g for 10 min at 4 °C (Centrifuge Hermle Z326, Labortechnik GmbH, Germany). The supernatant was then centrifuged again under the same conditions, and its absorbance measured at 290 nm. One PAL unit was defined as the amount of enzyme that produces 1 nmol of trans-cinnamic acid per hour. Results were expressed as U/mg protein (Ampofo & Ngadi, 2020).
Tyrosine ammonia-lyase assay. For the TAL activity, the reaction mixture contained 100 µL of enzyme extract (equivalent to 1 mg of protein) and 900 µL of 0.02 mM L-tyrosine and was incubated at 30 °C for 1 h. The reaction was stopped by adding 500 µL of 10% TCA, followed by centrifugation at 15,000 x g for 10 min at 4 °C (Centrifuge Hermle Z326, Labortechnik GmbH, Germany). The supernatant was then centrifuged again under the same conditions, and its absorbance measured at 310 nm. One TAL unit was defined as the amount of enzyme that produces 1 nmol of p-coumaric acid per hour. Results were expressed as U/mg protein (Ampofo & Ngadi, 2020).
2.4.4 Profile of phenolic compounds by UPLC-PDA-ESI-MS
A 250 mg sample of lyophilized material was weighed into a 15 mL conical tube, to which 5 mL of 50% ethanol was added. The extraction was carried out in an ultrasonic bath (Bransonic® Ultrasonic Cleaner; Model: 3510R-M) for 60 min. Afterward, the sample was centrifuged at 4,000 x g for 15 min at 4 °C, and the supernatant was recovered and filtered (0.45 µm PTFE). The extracts were stored in amber bottles at 4 °C. For instrumental analysis, a Waters Acquity H-Class UPLC (Waters, USA) was used, equipped with a quaternary pump (UPQSM), an automatic injector (UPPDALTC), and a PDA λ photodiode array detector (UPPDALTC). Chromatographic separation was achieved using a Waters Acquity UPLC BEH C18 column (1.7 μm, 100 x 2.1 mm ID) (Waters, USA). The PDA λ readout was performed in the 190–600 nm range.
The analytical absorbance response was recorded on two channels: 290 nm (channel A) and 350 nm (channel B). For mass spectrometry (MS/MS) analysis, a Waters Xevo TQ-S micro instrument was employed. Cone voltage (V) varied from 25 to 10 V for all samples. Mass spectra were recorded in full scan mode in negative ion mode, with a range from 50 to 2000 m/z. Data acquisition and processing were carried out using MassLynx V4.1 software (Waters, USA).
2.5 Determination of photosynthetic pigments
2.5.1 Chlorophylls
Chlorophyll content was determined according to Mariz-Ponte et al. (2018). An extract was prepared by mixing 0.5 g of freeze-dried sample with 80% acetone, followed by centrifugation at 10,000 × g for 10 min at 4 °C (Centrifuge Hermle Z326, Labortechnik GmbH, Germany). A 500 µL aliquot of the supernatant was collected, and absorbance was measured at 664 nm for Chlorophyll A and 647 nm for Chlorophyll B (Spectrophotometer UV/Vis, Perkin Elmer, USA). Chlorophyll content was estimated using the molar extinction coefficients for Chlorophyll A (Ɛ = 9.3) and Chlorophyll B (Ɛ = 47.5). Total chlorophyll was calculated by summing the values for both chlorophylls and was expressed as milligrams per gram of dry weight (mg chlorophyll/g DW).
2.5.2 Total carotenoids
Total carotenoid content was quantified spectro-photometrically according to Ercan et al. (2022). An extract was prepared by mixing 0.5 g of lyophilized material with 80% acetone, followed by centrifugation at 9,500 × g for 10 min at 4 °C (Centrifuge Hermle Z326, Labortechnik GmbH, Germany). A 500 µL aliquot was collected, and absorbance was measured at 664, 647, and 470 nm. Total carotenoids (Car) were expressed as µg carotenoids / g DW and calculated using the following equation:
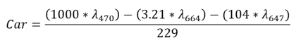
2.5.3 Identification and chromatographic quantifi-cation of carotenoids
Carotenoids were extracted from 250 mg of lyophilized sample by adding 50 mg of CaCO3 and 5 mL of a solvent mixture (MeOH/AcOEt/Et2O in a 1:1:1 v/v/v ratio). The mixture was shaken for 1 min and then centrifuged at 6,500 × g for 15 min. Afterward, the solvent was evaporated using nitrogen, and the residue was diluted in 2 mL of a MeOH/MTBE (1:1 v/v) mixture. Calibration curves for lutein, α-carotene, and β-carotene were prepared from standards ranging from 20 to 100 mg/kg in MeOH/MTBE (1:1 v/v). Both standards and samples were filtered using 0.2 µm filters (Millex-FG, PTFE) and analyzed by UPLC-PDA. Chromatographic separation was achieved using a Waters Acquity UPLC BEH C18, 1.7 μm, 100 x 2.1 mm ID column (Waters, USA), with MeOH (A) and 1 M MeOH:CH3COONH4 (70:30 v/v) (B) as the mobile phases. The elution program was isocratic, with a flow rate of 95% A and 5% B for 5 min. The analytical signal was measured at 430 nm. The results were expressed in µg/g DW.
2.6 Morphological properties
2.6.1 Phenotypic characterization
Freshly harvested broccoli sprouts were scanned at 600 dpi (24-bit color depth) using a HP OfficeJet Pro 8720 printer scanner. The color parameters (L*, a*, b*, H°, C*, and ∆E) of cotyledons, hypocotyls, and radicles, as well as the lengths of hypocotyls and radicles (Artés-Hernández et al., 2022), were determined using the ImageJ® software (Laboratory for Optical and Computational Instrumentation, University of Wisconsin, Madison, WI, USA).
2.6.2 Physicochemical parameters
Moisture Content. This analysis was performed according to AOAC method 23.003:2003. A 1 g sample was placed in a drying oven (SHEL-LAB, USA) at 100 ± 2 °C until a constant weight was achieved. Moisture content was then calculated using the following equation and expressed as a percentage (H%, were Wi is the initial weight and Wf is the final weight).
H (%)=((Wi-wf)/wi )*(100)
Dry matter content. This variable was determinate by the difference of the unit and previously calculated moisture content, according to the following equation.
SS (%)=(1-(%H)/100)*(100)
2.7 Statistical analysis
The data obtained were statistically analyzed using a one-way ANOVA. Additionally, a multiple comparison of means test was conducted using Tukey's method (p < 0.05), with Minitab® statistical software (Minitab, LLC., Pennsylvania, USA).
3. Results and discussion
3.1. Stress markers
H2O2 and proline as stress markers in 7-days broccoli sprouts obtained from UV-C seed primed are shown in Table 1. The results show that UV-C light treatment significantly increased H2O2 content by 1.7% and proline by 8.2% (p < 0.05). Among both stress markers, proline showed a greater response to treatment.
Previous studies have reported findings consistent with these results. For instance, Sen & Puthur (2021) observed significant increases in proline content in rice sprouts treated with UV-B radiation prior to germination (4 kJ/m2) although H2O2 levels did not differ significantly from the control. Similarly, Escobar-Hernández et al. (2024) reported signifi-cant increases in proline levels in tomato seedlings exposed to UV-A radiation (25 W, for 2 h) before seed germination, without significant changes in H2O2 content.
In this context, the results of our study suggest that UV-C pre-germinative treatments promote proline synthesis in broccoli sprouts, which could be related to the plant´s ability to maintain stable H2O2 concentration in the evaluated sprouts. In addition to its role as an osmolyte, proline plays key functions under stress conditions: it contributes to osmotic balance, stabilizes cell membranes and regulates ROS concentrations, keeping them within normal ranges and thus preventing oxidative damage in plants (Alagoz et al., 2023).
Table 1
Effects of UV-C radiation on stress markers and antioxidant enzymes in broccoli sprouts
Sample | Stress markers | Antioxidant enzymes |
H2O2 (mmol/g DW) | Proline (𝜇g/g DW) | SOD (U/mg protein) | APX (U/mg protein) | CAT (U/mg protein) |
Control | 13.0711 ± 0.3073b | 289.0750 ± 6.1133b | 3.1810 ± 0.0187a | 0.1213 ± 0.0145b | 4.8494 ± 0.7948b |
UV-C | 13.2930 ± 0.0615a | 312.6571 ± 7.2555a | 3.2692 ± 0.1609a | 0.1449 ± 0.0026a | 7.9570 ± 0.6756a |
Increment(§) | 1.7% | 8.2% | 2.77% | 19.49% | 64.08% |
Control represents untreated broccoli sprouts, while UV-C corresponds to sprouts treated with UV-C light (60 kJ/m2, 8 cm of distance).
Different letters within each row indicate statistically significant differences (p < 0.05).
§, Percentage increment of response to UV-C treatment.
3.2. Stress indicator enzymes
Table 1 shows the effect of UV-C light treatment on the activity of SOD, CAT and APX in broccoli sprouts. The results indicate that UV-C treatment in broccoli seeds did not significantly affect SOD activity in broccoli sprouts, despite a slight increase of 2.77% compared to the control (p > 0.05). In contrast, APX and CAT activities showed significant increases of 19.49% and 64%, respectively (p < 0.05). It is noteworthy that SOD serves as the first line of antioxidant defense in plants, playing a pivotal role in the removal of superoxide anion by converting it into H₂O₂. Subsequently, H₂O₂ is broken down into water, oxygen, and other harmless products by the CAT and APX enzymes (Jhanji et al., 2024).
These findings suggest that UV-C radiation treatment induce oxidative stress in plants, which stimulates the activity of APX and CAT enzymes to enable the rapid elimination of H₂O₂ generated as a result of the applied treatment. Similar results were reported by Thomas et al. (2020), who observed increases of 60% and 40% in CAT and APX activity, respectively, in rice seeds exposed to UV-B radiation (4 kJ/m²), with no significant changes in SOD activity. Conversely, Piechowiak (2024) reported significant increases in SOD and CAT gene expression by 42% and 13%, respectively, in broccoli seeds treated with UV-C light 48 h after germination. This study also highlighted significant reductions in ROS production, attributing these effects to the activation of the antioxidant enzymatic system.
3.3. Bioactive compounds and related enzymes
Regarding the effect of UV-C radiation on broccoli seeds, this treatment significantly increased (p < 0.05) the TF content in the sprouts. Although the TPC content showed a 7.6% increase, this change was not significant (Fig. 1A). In contrast, TF content showed a notable increase of 20.52% (Figure 1B). In terms of the activity of precursor enzymes involved in the synthesis of these biocompounds, PAL and TAL, no significant differences (p > 0.05) were observed in UV-C-treated sprouts (Figures 1C and 1D, respectively).
The relationship between the enzymes PAL and TAL and their influence on the synthesis of phenolic compounds has been widely documented. Piechowiak (2024) reported that exposing pregerminated broccoli seeds (48 h of germination) to UV-C radiation (2.3 W/m², 50 cm distance, 5 min) led to 6% and 43% increase in TPC and TF content, respectively, three days after treatment. This effect was attributed to increased gene expression of proteins involved in the phenylpropanoid pathway, such as PAL and chalcone synthase (CHS), induced by UV-C radiation.
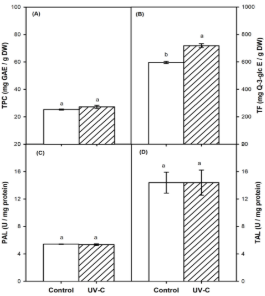
Figure 1. Bioactive compounds and Biocompound-Related Enzyme Activity in Broccoli Sprouts. (A) Total Phenolic Compounds (TPC), (B) Total Flavonoids (TF), (C) Phenylalanine Ammonia-Lyase (PAL) and (D) Tyrosine Ammonia-Lyase (TAL). Control represents untreated broccoli sprouts, while UV-C corresponds to sprouts treated with UV-C light (60 kJ/m2, 8 cm of distance). Different letters within each plot indicate significant differences (p < 0.05).
In the present study, similar patterns were observed, where UV-C radiation treatment enhanced total phenolic content (TPC), and particularly total flavonoid content (TF), despite no significant changes in the activity of phenylalanine ammonia-lyase (PAL) and tyrosine ammonia-lyase (TAL). Comparable results were reported by Zhu et al. (2020) in germinated peanut seeds (48 h of germination) treated with UV-C radiation (180 µW/cm² for 8 h/day over 8 days). These authors found that PAL and TAL activities increased significantly four days after treatment but subsequently declined, returning to control levels by the time of harvest.
Similarly, Tian et al. (2024) reported significant increases in TPC and TF content (40% and 73%, respectively) in buckwheat sprouts exposed to UV-B radiation during the first 72 hours of germination (λ = 313 nm, 30 µmol m⁻² s⁻¹ for 8 h per day), which were accompanied by enhanced activities of key enzymes in the phenylpropanoid pathway, including PAL, 4CL, C4H, and CHI. However, a marked reduction in enzyme activities, particularly PAL, was observed after 120 hours, suggesting a transient induction modulated by the duration of the stress stimulus. Collectively, these findings, along with the results of the present study, indicate that although PAL plays a central role in the biosynthesis of phenolic compounds, its activity may be temporally restricted and not necessarily sustained throughout the sprouting period. Therefore, the accumulation of total phenolic compounds (TPC) and total flavonoids (TF) could result from the early activation of PAL and TAL during the initial stages of germination. Additionally, the nature and intensity of applied stress, and alternative regulatory pathways and other enzymes may be involved in the biosynthetic response induced by UV-C priming treatment (Verdaguer et al., 2017).
Using liquid chromatography, seven phenolic compounds (M-1 to M-7) were detected in broccoli sprouts; however, only five of these compounds were successfully identified (Table 2). Among the identified compounds M-1 was tentatively characterized as 1-O-sinapoyl-β-D-glucose (385 m/z), and M-4 as sinapic acid (223 m/z). Furthermore, M-5 and M-6 were identified as 1-O,2-O-bis[(E)-3,5-dimethoxy-4-hydroxycinnamoyl]-6-O(β-D-glucopyranosyl)-β-D-glucopyranose and 1,2-di-O-sinapoyl-β-D-glucose, respectively. While M-2 and M-3 could not be fully characterized. However, their fragmentation patterns suggest they are derivatives of sinapic acid.
These findings are consistent with those from Moreira-Rodríguez et al. (2017), who identified 22 phenolic compounds in broccoli sprouts treated postharvest with UV-A and UV-B radiation, about 12 of which were sinapic acid derivatives. The increased content of some of these compounds has been attributed to the expression of ethylene synthesis-related genes in plants subjected to mechanical stress. In the case of UV-C-treated sprouts, it is likely that this treatment stimulates the synthesis of plant growth regulators that act as signaling molecules to regulate the expression of stress-associated genes, including those involved in phenolic compound biosynthesis. Additionally, hydroxycinnamic acid derivatives, such as sinapic acid esters, play a crucial role in protecting the leaf epidermis from UV radiation. These compounds are synthesized and incorporated into the epidermis, where they contribute to UV-B tolerance by reducing radiation penetration into plant tissues (Moreira-Rodriguez et al., 2017).
Table 2
Chromatographic identification of phenolic compounds in broccoli sprouts
C | RT (min) | λmax | MI ([M - H]-) m/z | Tentative identification | MW | Fragments m/z |
M-1 | 9.51 | 200, 225, 238, 330 | 385 | 1-O-Sinapoyl-beta-D-glucose | 386.3 | 223, 205, 190, 175, 147, 119, 91 |
M-2 | 10.51 | 200, 268, 329 | 783 | Unknown | NA | 680, 599, 467, 367, 299, 284, 271, 255, 223, 205, 190, 149, 119, 97 |
M-3 | 10.85 | 200, 268, 329 | 672 | Unknown | NA | 591, 284, 255, 227, 223, 205, 190, 175, 162, 149, 119, 97 |
M-4 | 11.51 | 200, 224, 238, 323 | 223 | Sinapic acid | 224.2 | 208, 193, 164, 149, 121 |
M-5 | 13.30 | 199, 225, 238, 329 | 753 | 1-O, 2-O-Bis[(E)-3,5-dimethoxy-4-hydroxycinnamoyl]-6-O-(beta-D-glucopyranosyl) -beta-D-glucopyranose | 754.7 | 529, 265, 247, 223, 205, 190, 175, 164, 149, 119 |
M-6 | 16.18 | 222, 329 | 591 | 1,2-di-O-sinapoyl -beta-D-glucose | 592.5 | 367, 223, 205, 190, 175, 164, 149 |
M-7 | 16.77 | 200, 226, 237, 326 | 959 | 1,2,2” Trisinapoylgentiobioside | 960.9 | 735, 529, 511, 487, 469, 427, 385, 265, 246, 223, 205, 190, 175, 164, 149, 119 |
Note: The table shows the sample code (C), retention time (RT), maximum wavelength (λmax), molecular ion (MI), molecular weight of identified phenolic compounds (MW) and fragments of detected compounds.
3.4. Photosynthetic pigments
The levels of chlorophyll A, chlorophyll B, total chlorophyll, and total carotenoids in broccoli sprouts were analyzed to assess the impact of UV-C light treatment (Table 3). The results revealed that exposure to UV-C light negatively affected these photosynthetic pigments. Chlorophyll A levels decreased slightly by 1.38%, whereas chlorophyll B experienced a more pronounced reduction (21%). Similarly, total chlorophyll content decreased by 1.62% compared to untreated sprouts (p < 0.05).
Total carotenoids were also significantly reduced by UV-C treatment, showing a decline of 13.05% (p < 0.05; Table 3). Chromatographic analysis of specific pigments, such as β-carotene and lutein, revealed reductions of 15.22% and 16.75%, respectively (p < 0.05; Table 3). These decreases correspond with the overall decline in total carotenoids observed in UV-C-treated broccoli sprouts, highlighting the potential role of carotenoids in counteracting UV-C-induced oxidative stress. Carotenoids may help mitigate the damaging effects of ROS generated under such conditions (Gómez-Sagasti et al., 2023).
The findings of the present study are consistent with those reported by Santin et al. (2022), who observed significant decreases in carotenoid and chlorophyll contents in flaxseed sprouts subjected to UV-B radiation 72 hours after seed germination (1.33 W/m² for 24 h). This treatment resulted in pronounced reductions in the concentrations of violaxanthin, antheraxanthin, lutein, and chlorophyll a, which decreased by 38%, 67%, 26%, and 22%, respectively, compared to the control. Similarly, Escobar-Hernández et al. (2024) reported slight decreases in total chlorophyll, chlorophyll A, and chlorophyll B levels in chili bell pepper seedlings exposed to UV-A radiation. These changes were accompanied by a notable reduction of approximately 16% in β-carotene content relative to the control (Table 3).
Some authors suggest that, under high-energy light conditions, plants may redirect their biosynthetic machinery toward the production and accumula-tion of more effective UV-screening and ROS-scavenging compounds, such as flavonoids, to better acclimate to UV-induced stress. This adaptive strategy may lead to a reduction in the biosynthesis of photosynthetic pigment (Santin et al., 2022).
On the other hand, the decrease in chlorophyll levels under stress conditions is often associated with pigment degradation and impairments in photosynthetic processes (Terletskaya et al., 2017).
3.5 Morphological characteristics
The effects of UV-C radiation exposure prior to germination on the morphological and physicochemical parameters of broccoli sprouts are summarized in Figure 2, where a significant 10% increase in the moisture content of broccoli sprouts was observed, accompanied by a corresponding decrease in the content of dry soluble solids (p < 0.05).
Additionally, the fresh weight-to-dry weight ratio (FW/DW) increased by 13% (p < 0.05). In terms of root length, broccoli sprouts exposed to UV-C light showed a significant increase (p < 0.05), whereas no
significant differences were observed in hypocotyl length. Shoot and leaf morphology, which are critical determinants of light absorption and photosynthetic efficiency, were indirectly impacted. UV-C radiation negatively affected chlorophyll content, which may explain the observed decrease in the dry weight of sprouts. This aligns with previous studies that identify chlorophyll content as a key parameter for assessing plant growth. Conversely, the fresh weight, commonly used as a yield metric, appeared to benefit from UV-C exposure, as suggested by the results of this study.
Regarding color parameters (Table 4), no significant differences were detected in L*, a*, b*, C*, and H° values for cotyledons and hypocotyls of broccoli sprouts from UV-C-treated seeds. However, the roots exhibited an increase in b*, C, and H° values, along with a more pronounced color difference (ΔE = 2.99) compared to cotyledons (ΔE = 1.89) and hypocotyls (ΔE = 0.89). However, such differences were not visually perceptible.
Table 3
Effects of UV-C radiation on photosynthetic pigments and carotenoids in broccoli sprouts
Sample | Photosynthetic pigments | Carotenoids |
Chlorophyll A(†) (𝜇g/g DW) | Chlorophyll B(†) (𝜇g/g DW) | Total carotenoids(†) (𝜇g/g DW) | β-carotenoid(§) (𝜇g/g DW) | Lutein(§) (mg/g DW) |
Control | 12.5680 ± 0.0481a | 3.2874 ± 0.0177a | 142.4947 ± 0.9435a | 25.81 ± 3.94a | 239.45 ± 8.56a |
UV-C | 12.3939 ± 0.0183b | 2.5970 ± 0.0376b | 123.9065 ± 0.9663b | 21.88 ± 1.99a | 199.33 ± 7.69b |
Decrement(&) | 1.38% | 21% | 13.05% | 15.22% | 16.75% |
Control represents untreated broccoli sprouts, while UV-C corresponds to sprouts treated with UV-C light (60 kJ/m2, 8 cm of distance). Different letters within each row indicate statistically significant differences (p < 0.05).
† biocompounds measured by spectrophometric techniques.
§ biocompounds measured by chromatographic techniques.
& percentage decrement of response to UV-C treatment.
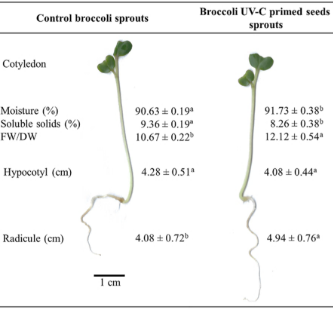
Figure 2. Broccoli UV-C primed seed effect on the morphological and physicochemical parameters on broccoli sprouts. Average values for root and hypocotyl (n=10); moisture content, dry weight percentage, and FW/DW ratio (n = 3), ± standard deviation. Different letters between columns indicate significant differences (p < 0.05).
Table 4
Effect of UV-C radiation on color parameters in broccoli sprouts
Color parameters | Sample | Physiological structures of the sprouts |
Cotyledons | Hypocotyl | Root |
L* | C | 34.80 ± 0.66ª | 83.24 ± 0.79ª | 92.83 ± 1.52ª |
UV-C | 36.25 ± 1.24a | 82.69 ± 0.50a | 91.46 ± 0.34a |
a* | C | -20.85 ± 1.05ª | -10.75 ± 0.80ª | -1.46 ± 0.25ª |
UV-C | -21.21 ± 0.98a | -11.02 ± 1.13a | -1.41 ± 0.15a |
b* | C | 18.71 ± 1.76ª | 17.89 ± 0.83ª | 3.47 ± 0.91b |
UV-C | 19.88 ± 0.99a | 18.60 ± 0.73a | 6.18 ± 0.79a |
H° | C | 137.94 ± 0.21ª | 121.02 ± 0.02ª | 111.31 ± 2.12ª |
UV-C | 136.14± 1.00a | 120.18 ± 0.65a | 104.29 ± 2.03b |
C* | C | 28.04 ± 1.53ª | 20.87 ± 0.96ª | 3.93 ± 0.23b |
UV-C | 29.08 ± 1.24a | 21.63 ± 0.97a | 5.74 ± 0.82a |
Color coding | C | #355a32 | #c5d5ad | #eaebe4 |
UV-C | #385e34 | #c3d3aa | #e8e7bd |
∆E | C | 1.89 | 0.94 | 2.99 |
UV-C |
Mean value (n = 10) ± standard deviation. CIELab scale color parameters. Different letters, per color parameter and between treatments, indicate significant difference (p < 0.05).
Although studies on the impact of UV-C radiation prior to germination on sprout color are limited, similar findings have been reported in other contexts. For instance, Bilgin and Akocak (2024) found that different post-harvest UV-C radiation treatments in cherries did not result in significant changes in color parameters.
4. Conclusions
UV-C radiation pre-germination stage, induces oxidative processes capable of activating defense mechanisms, including proline synthesis and the activity of antioxidant enzymes such as catalase and ascorbate peroxidase in broccoli sprouts. These responses may be linked to the enhanced production of bioactive secondary metabolites, such as phenolic compounds and flavonoids, underscoring the potential of UV-C radiation to increase the bioactive content of fresh broccoli sprouts. Although adverse effects on photosynthetic pigments were observed, no significant changes in morphological or color parameters were detected. This indicates that the application of UV-C radiation (60 kJ/m2, 8 cm of distance) during the pre-germination stage is an effective and practical strategy.
Overall, this approach presents a promising tool for enhancing the nutraceutical quality of sprouts. Future studies should focus on optimizing UV-C radiation conditions to maximize antioxidant benefits while minimizing potential adverse effects. Additionally, the applicability of this technique should be evaluated across different crops and environmental conditions.
Authors' contributions
M. A. C. Centeno-Rodriguez carried out the experiments and wrote the manuscript; J. E. Ruiz-Nieto, L. E. Casados-Vazquez, M. E. Sosa-Morales, A. Ceron-Garcia supervised the work, A. Ceron-Garcia, J. A. Gomez-Salazar, M. A. Martinez-Tellez edited the manuscript, formal analysis, A. Ceron-Garcia conceptualization, review editing, formal analysis.
Acknowledgements
Authors thank CONAHCYT (Consejo Nacional de Humanidades, Ciencias y Tecnologías) for the financial support for PhD studies for author M.A.C. Centeno-Rodríguez.
ORCID
M. A. C. Centeno-Rodríguez https://orcid.org/0000-0001-9121-4466
J. A. Gómez-Salazar https://orcid.org/0000-0002-9088-4721
J. E. Ruiz-Nieto https://orcid.org/0000-0002-3293-8066
M. A. Martínez-Téllez https://orcid.org/0000-0003-2667-5455
L. E. Casados-Vázquez https://orcid.org/0000-0001-8132-5488
M. E. Sosa-Morales https://orcid.org/0000-0002-1197-2572
A. Cerón-García https://orcid.org/0000-0001-6905-6166
References
Abellán, Á., Domínguez-Perles, R., Moreno, D. A., & García-Viguera, C. (2019). Sorting out the value of cruciferous sprouts as sources of bioactive compounds for nutrition and health. Nutrients, 11(2), 429. https://doi.org/10.3390/nu11020429
Alagoz, S. M., Lajayer, B. A., & Ghorbanpour, M. (2023). Proline and soluble carbohydrates biosynthesis and their roles in plants under abiotic stresses. In Plant Stress Mitigators (pp. 169-185). Academic Press. https://doi.org/10.1016/b978-0-323-89871-3.00027-6
Ampofo, J. O., & Ngadi, M. (2020). Ultrasonic assisted phenolic elicitation and antioxidant potential of common bean (Phaseolus vulgaris) sprouts. Ultrasonics Sonochemistry, 64, 104974. https://doi.org/10.1016/j.ultsonch.2020.104974
Artés–Hernández, F., Miranda-Molina, F. D., Klug, T. V., & Martínez–Hernández, G. B. (2022). Enrichment of glucosinolate and carotenoid contents of mustard sprouts by using green elicitors during germination. Journal of Food Composition and Analysis, 110, 104546. https://doi.org/10.1016/j.jfca.2022.104546
Bates L. S., Waldren R. P., and I. D. Teare. (1973). Rapid determination of free proline for water-stress studies. Plant Soil, 39, 205-207. https://doi.org/10.1007/bf00018060
Bilgin, A. B., & Akocak, P. B. (2024). Improved postharvest quality attributes of sweet and sour cherry by using low pressure UV-C versus light-emitting diodes (LEDs). Scientia Horticulturae, 327, 112860. https://doi.org/10.1016/j.scienta.2024.112860
Cao, N., Fan, J., Yang, Z., Hao, L., Kang, Q., Liu, X., & Lu, J. (2020). Increasing Antioxidant Potentials of Mung Bean Sprouts. Current Topics in Nutraceutical Research, 18(1), 75-82.
Ercan, I., Tombuloglu, H., Alqahtani, N., Alotaibi, B., Bamhrez, M., et al. (2022). Magnetic field effects on the magnetic properties, germination, chlorophyll fluorescence, and nutrient content of barley (Hordeum vulgare L.). Plant Physiology and Biochemistry, 170, 36-48. https://doi.org/10.1016/j.plaphy.2021.11.033
Escobar-Hernández, D. I., González-García, Y., Olivares-Sáenz, E., & Juárez-Maldonado, A. (2024). Seedling priming with UV-A radiation induces positive responses in tomato and bell pepper plants under water stress. Scientia Horticulturae, 332, 113235. https://doi.org/10.1016/j.scienta.2024.113235
García‐Mosqueda, C., Cerón‐García, A., León‐Galván, M. F., Ozuna, C., López‐Malo, A., & Sosa‐Morales, M. E. (2023). Changes in phenolics and flavonoids in amaranth and soybean sprouts after UV‐C treatment. Journal of Food Science, 88(4), 1280-1291. https://doi.org/10.1111/1750-3841.16527
Gómez-Sagasti, M. T., López-Pozo, M., Artetxe, U., Becerril, J. M., Hernández, A., García-Plazaola, J. I., & Esteban, R. (2023). Carotenoids and their derivatives: A “Swiss Army knife-like” multifunctional tool for fine-tuning plant-environment interactions. Environmental and Experimental Botany, 207, 105229. https://doi.org/10.1016/j.envexpbot.2023.105229
Hernández-Aguilar, C., Dominguez-Pacheco, A., Tenango, M. P., Valderrama-Bravo, C., Hernández, M. S., Cruz-Orea, A., & Ordonez-Miranda, J. (2021). Characterization of bean seeds, germination, and phenolic compounds of seedlings by UV-C radiation. Journal of Plant Growth Regulation, 40(2), 642-655. https://doi.org/10.1007/s00344-020-10125-0
Jhanji, S., Goyal, E., Chumber, M., & Kaur, G. (2024). Exploring fine tuning between phytohormones and ROS signaling cascade in regulation of seed dormancy, germination and seedling development. Plant Physiology and Biochemistry, 108352. https://doi.org/10.1016/j.plaphy.2024.108352
Ji, H., Tang, W., Zhou, X., & Wu, Y. (2016). Combined effects of blue and ultraviolet lights on the accumulation of flavonoids in Tartary buckwheat sprouts. Polish Journal of Food and Nutrition Sciences, 66(2), 93-98. https://doi.org/10.1515/pjfns-2015-0042
Khanam, U. K. S., Oba, S., Yanase, E., & Murakami, Y. (2012). Phenolic acids, flavonoids and total antioxidant capacity of selected leafy vegetables. Journal of Functional Foods, 4(4), 979-987. https://doi.org/10.1016/j.jff.2012.07.006
Li, C., Song, S., Yue, Y., & Liu, H. (2025). Preharvest CaCl2-HCl electrolyzed water treatment maintained the quality of broccoli sprouts during storage. Journal of Future Foods, 5(2), 208-217. https://doi.org/10.1016/j.jfutfo.2024.05.010
Li, L., Ma, P., Nirasawa, S., & Liu, H. (2024). Formation, immunomo-dulatory activities, and enhancement of glucosinolates and sulforaphane in broccoli sprouts: a review for maximizing the health benefits to human. Critical Reviews in Food Science and Nutrition, 64(20), 7118-7148. https://doi.org/10.1080/10408398.2023.2181311
Mariz-Ponte, N., Mendes, R.J., Sario, S., Melo, P., & Santos, C. (2018). Moderate UV-A supplementation benefits tomato seed and seedling invigoration: a contribution to the use of UV in seed technology. Scientia Horticulturae, 235, 357-366. https://doi.org/10.1016/j.scienta.2018.03.025
Moreira-Rodríguez, M., Nair, V., Benavides, J., Cisneros-Zevallos, L., & Jacobo-Velázquez, D. A. (2017). UVA, UVB light doses and harvesting time differentially tailor glucosinolate and phenolic profiles in broccoli sprouts. Molecules, 22(7), 1065. https://doi.org/10.3390/molecules22071065
Ozuna, C., Cerón-García, A., Sosa-Morales, M. E., Salazar-Goméz, J. A., León-Galván, M. F., Abraham-Juárez, M. R. (2018). Electrically induced changes in amaranth seed enzymatic activity and their effect on bioactive compounds content after germination. Journal of Food Science and Technology, 55, 648-657. https://doi.org/10.1007/s13197-017-2974-0
Paucar-Menacho, L.M., Peñas, E., Dueñas, M., Frias, J., & Martínez-Villaluenga, C. (2017). Optimizing germination conditions to enhance the accumulation of bioactive compounds and the antioxidant activity of kiwicha (Amaranthus caudatus) using response surface methodology. LWT-Food Science and Technology, 76, 245-252. https://doi.org/10.1016/j.lwt.2016.07.038
Piechowiak, T. (2024). Elucidation of the mechanism of elicitation of edible sprouts using UV-C radiation. Biocatalysis and Agricultural Biotechnology, 103081. https://doi.org/10.1016/j.bcab.2024.103081
Piechowiak, T., & Balawejder, M. (2025). Succinic acid treatment enhances energy metabolism and antioxidant biosynthesis in radish sprouts. Journal of Biotechnology, 404, 144-151. https://doi.org/10.1016/j.jbiotec.2025.04.017
Rizi, M.R., Azizi, A., Sayyari, M., Mirzaie-Asl, A., & Conti, L. (2021). Increased phenylpropanoids production in UV-B irradiated Salvia verticillate as a consequence of altered genes expres-sion in young leaves. Plant Physiology and Biochemistry, 167, 174-184. https://doi.org/10.1016/j.plaphy.2021.07.037
Santin, M., Sciampagna, M. C., Mannucci, A., Puccinelli, M., Angelini, L. G., et al. (2022). Supplemental UV-B exposure influences the biomass and the content of bioactive compounds in Linum usitatissimum L. sprouts and microgreens. Horticulturae, 8(3), 213. https://doi.org/10.3390/horticulturae8030213
Sen, A., & Puthur, J. T. (2021). Halo- and UV-B priming-mediated drought tolerance and recovery in rice seedlings. Plant Stress, 2, 100011. https://doi.org/10.1016/j.stress.2021.100011
Slinkard, K., & Singleton, V. L. (1977). Total phenol analysis: auto-mation and comparison with manual methods. American Journal of Enology and Viticulture, 28(1), 49-55. https://doi.org/10.5344/ajev.1977.28.1.49
Terletskaya, N., Zobova, N., Stupko, V., & Shuyskaya, E. (2017). Growth and photosynthetic reactions of different species of wheat seedlings under drought and salt stress. Periodicum Biologorum, 119(1), 37-45. https://doi.org/10.18054/pb.v119i1.4408
Thomas, T. D., Dinakar, C., & Puthur, J. T. (2020). Effect of UV-B priming on the abiotic stress tolerance of stress-sensitive rice seedlings: Priming imprints and cross-tolerance. Plant Physiology and Biochemistry, 147, 21-30. https://doi.org/10.1016/j.plaphy.2019.12.002
Tian, X., Hu, M., Yang, J., Yin, Y., & Fang, W. (2024). Ultraviolet-B Radiation Stimulates Flavonoid Biosynthesis and Antioxidant Systems in Buckwheat Sprouts. Foods, 13(22), 3650. https://doi.org/10.3390/foods13223650
Urban, L., Charles, F., de Miranda, M.R.A., & Aarrouf, J. (2016). Understanding the physiological effects of UV-C light and exploiting its agronomic potential before and after harvest. Plant Physiology and Biochemistry, 105, 1-11. https://doi.org/10.1016/j.plaphy.2016.04.004
Verdaguer, D., Jansen, M.A., Llorens, L., Morales, L.O., & Neugart, S. (2017). UV-A radiation effects on higher plants: Exploring the known unknown. Plant science, 255, 72-81. https://doi.org/10.1016/j.plantsci.2016.11.014
Wang, M., Li, Y., Yang, Y., Tao, H., Mustafa, G., et al. (2023). Biofortification of health-promoting glucosinolates in cruciferous sprouts along the whole agro-food chain. Trends in Food Science & Technology, 104164. https://doi.org/10.1016/j.tifs.2023.104164
Xia, Y., Li, M. Y., Wadood, S. A., Hong, H. J., Liu, Y., et al. (2024). Identification of volatile and flavor metabolites in three varieties of broccoli sprouts. Food Chemistry: X, 24, 101862. https://doi.org/10.1016/j.fochx.2024.101862
Zhu, T., Yang, J., Zhang, D., Cai, Q., Zhou, D., et al. (2020). Effects of white LED light and UV-C radiation on stilbene biosynthesis and phytochemicals accumulation identified by UHPLC–MS/MS during peanut (Arachis hypogaea L.) germination. Journal of Agricultural and Food Chemistry, 68(21), 5900-5909. https://doi.org/10.1021/acs.jafc.0c01178
]