Agronomic, physiological, and phytochemical responses of physalis to pre- and post-emergence herbicides
Anderson Luis Nunes Gabardo1, 3 *; Denise Bilibio¹; Wagner Priamo¹; Serleni Geni Sossmeier¹; Rubens Polito2 ; Rafaela Cinelli3
¹ Curso de Agronomia - Instituto Federal de Educação, Ciência e Tecnologia do Rio Grande do Sul, Sertão, Rio Grande do Sul, Brasil.
² Programa de Pós-graduação em Fitossanidade da Universidade Federal de Pelotas, Pelotas, Rio Grande do Sul, Brasil.
³ Programa de Pós-graduação em Agronomia da Universidade Tecnológica Federal do Paraná, Pato Branco, Paraná, Brasil.
* Corresponding author: anderson.gabardo@sertao.ifrs.edu.br (A. L. N. Gabardo).
Received: 9 April 2025. Accepted: 3 August 2025. Published: 18 August 2025.
Abstract
Cape gooseberry (Physalis peruviana L.) is a fruit crop with increasing economic and functional relevance, yet limited research exists on weed management practices for this species. This study aimed to evaluate the agronomic, physiological, and phytochemical responses of P. peruviana to various pre- and post-emergence herbicides under greenhouse and field conditions. Two biotypes were used to assess selectivity and crop tolerance to thirteen post-emergence and two pre-emergence herbicides. Post-emergence trials revealed that chlorimuron, fomesafen, and the mixture atrazine + simazine significantly reduced plant height and caused high phytotoxicity, especially under field conditions. Conversely, quizalofop, clethodim, fluazifop, and clodinafop (ACCase inhibitors) showed excellent selectivity and maintained yield levels comparable to the control. Pre-emergence applications of S-metolachlor exhibited minimal effects on plant growth and effectively reduced weed density, while imazaquin caused a dose-dependent reduction in plant height and yield, particularly in one biotype. Phenolic compound analysis indicated that both herbicide application and weed presence influenced fruit quality. Plants grown under weed-free conditions presented the highest total phenolic content, while high weed pressure or herbicide injury reduced phenolic accumulation, especially in biotype 2. The results demonstrate that while some herbicides pose risks to P. peruviana development, others offer promising weed control options with minimal impact on crop performance and fruit quality. These findings contribute to the development of safe and effective weed management strategies for this emerging crop.
Keywords: Herbicide selectivity; Fruit quality; Physalis peruviana; herbicides; phytotoxicity.
DOI: https://doi.org/10.17268/sci.agropecu.2025.043
Cite this article:
Gabardo, A. L. N., Bilibio, D., Priamo, W., Sossmeier, S. G., Polito, R., & Cinelli, R. (2025). Agronomic, physiological, and phytochemical responses of physalis to pre- and post-emergence herbicides. Scientia Agropecuaria, 16(4), 565-576.
1. Introduction
Physalis peruviana L., commonly known as Cape gooseberry or goldenberry, is a fruit species of the Solanaceae family that has gained growing agronomic and pharmacological interest. Its cultivation has expanded across tropical and subtropical regions, driven by the increasing demand for fruits with functional properties, high antioxidant content, and economic potential in specialized markets (Muniz et al., 2015). Despite its added value, the crop still lacks advances in developing specific management practices, including cultivar selection and weed control strategies that ensure productivity and fruit quality.
The use of post-emergence herbicides in Solanaceae crops such as tomato, eggplant, and pepper is well documented, but studies involving Physalis spp. are still limited. Herbicides such as fomesafen, chlorimuron, and bentazon have been tested in Physalis angulata and Solanum lycopersicum, showing considerable variability in selectivity and phytotoxicity (Chiconi et al., 2022; Gnanasekaran, 2022). These variations underscore the importance of evaluating herbicide safety for each species and growing condition, as inappropriate use can severely impair crop development.
Regarding pre-emergence management, herbicides such as S-metolachlor and imazaquin have been highlighted for their broad-spectrum weed control and potential selectivity in broadleaf crops. Imazaquin has significantly inhibited early growth in tomatoes, while S-metolachlor has demonstrated more excellent selectivity in Solanaceae (Dittmar et al., 2012; Gazola et al., 2021). Preliminary studies suggest that, in P. peruviana, these molecules may be used effectively for early-season weed control without compromising crop development.
Weed control is a critical factor for the successful cultivation of Physalis. Competition for light, water, and nutrients can drastically reduce growth and yield, especially during early developmental stages (Nosratti et al., 2020). Integrated strategies combining selective herbicides, cultural practices, and weed monitoring are essential to ensure healthy plant growth and efficient field establishment (Coleman et al., 2024; Uljol et al., 2018).
In addition to productivity aspects, the functional quality of the fruits is directly influenced by crop management, including weed pressure and herbicide application. Phenolic compounds such as flavonoids and phenolic acids are recognized for their antioxidant and pharmacological properties, serving as essential indicators of fruit quality (Guiné et al., 2020; Yari et al., 2025). Therefore, this study aimed to evaluate the selectivity of pre- and post-emergence herbicides in Physalis peruviana and their effects on fruit yield and phenolic compound content.
2. Methodology
The experiments were conducted at the Federal Institute of Education, Science and Technology of Rio Grande do Sul (IFRS) – Sertão Campus, located at 28º02'57'' S, 52º16'22'' W, at an altitude of 748 m, during the 2013/2014 growing season. The local climate is classified as Cfa according to Köppen, with an average annual rainfall of 1,800 mm and average minimum and maximum temperatures of 13.2 °C and 23.6 °C, respectively. The soil in the experimental area is classified as a typical dystrophic Red Nitosol (Santos et al., 2018).
Physalis biotypes
One or two Physalis peruviana L. biotypes were used in the experiments. Biotype 1 originated from Sertão (RS), and biotype 2 from Capelinha (MG). In the season before the experiments, plants were acclimated to the experimental site and used for seed multiplication. Seeds were extracted from mature fruits (with calyx ranging from green-yellow to straw-yellow), cleaned, shade-dried, and stored in paper bags in a dry, dark place at room temperature.
Effect of post-emergence herbicides on greenhouse conditions
The greenhouse experiment followed a completely randomized design, arranged in a 2 × 13 factorial scheme (two biotypes × 13 herbicide treatments – Table 1), with three replications. Seeds were sown in 128-cell polyethylene trays filled with commercial peat-based substrate (Carolina Padrão®) and maintained in a mist chamber with overhead irrigation (15 min, three times per day). When seedlings reached approximately 10 cm in height, they were transplanted into plastic pots (0.0003 m³ of substrate) and transferred to a greenhouse with controlled temperature and photoperiod.
Herbicide applications were performed when plants had 4 to 6 fully expanded leaves, using a boom sprayer with flat-fan nozzles (110.015) spaced 0.50 m apart, delivering a spray volume of 120 L ha⁻¹. Plant height was measured at 0, 35, and 45 days after application (DAA), and shoot dry mass was assessed at 45 DAA. Height was recorded with a 30-cm ruler from the base to the apex. Dry mass was obtained by drying plant material at 65 °C for 72 hours in a forced-air circulation oven (Marconi MA 35/5) and weighing it with a semi-analytical balance (Shimadzu UX2200H, 0.01 g precision).
Table 1
Commercial names, formulations, concentrations, rates, and manufacturers of the herbicides used in the greenhouse and field postemergence experiments
Trade name | Formulation 1 | [ ]2 (g L⁻¹ or g kg⁻¹) | Rate (c.p. ha⁻¹) | Active ingredient | Rate (g a.i. ha⁻¹) | Manufacturer |
Control | -- | -- | -- | -- | -- | -- |
Flex | SL | 250 | 1000 | Fomesafen | 250 | Syngenta |
Targa Max | EC | 50 | 1500 | Quizalofop-p-ethyl | 75 | Ihara |
Select 240 EC | EC | 240 | 400 | Clethodim | 96 | UPL |
Topik 240 EC | EC | 240 | 150 | Clodinafop-propargyl | 36 | Syngenta |
Fusilade | EC | 250 | 1000 | Fluazifop-p-butyl | 250 | Syngenta |
Facet | SC | 500 | 750 | Quinclorac | 375 | BASF |
Clorim | WG | 250 | 80 | Chlorimuron-ethyl | 20 | UPL |
Hussar | WG | 50 | 70 | Iodosulfuron-methyl | 3.5 | Bayer |
Sanson 40 SC | SC | 40 | 1250 | Nicosulfuron | 50 | ISK Biosc. |
Basagran 600 | SL | 600 | 1200 | Bentazon | 720 | BASF |
Sencor 480 | SC | 480 | 300 | Metribuzin | 144 | Bayer |
Primatop SC | SC | 250 + 250 | 3000 | Atrazine + simazine | 750 + 750 | Syngenta |
1 SL = Soluble Concentrate; EC = Emulsifiable Concentrate; SC = Suspension Concentrate; WG = Water-Dispersible Granule.
2 [ ] Concentration.
Effect of post-emergence herbicides on field conditions
The field experiment followed a randomized block design with four replications. No differences between biotypes were observed in the greenhouse, so only biotype 1 was used. The same 12 herbicides tested in the greenhouse (Table 1) were evaluated, along with hand-weeded and non-weeded controls, totaling 56 plots (6 × 1 m), with assessments performed on the four central plants.
Fertilization was based on recommendations for tomato crops (estimated yield of 10 t ha⁻¹). Planting holes (30 × 30 × 30 cm) were prepared 15 days before transplanting and received 150 g of NPK 04-14-08 incorporated into the soil. Transplanting was carried out when seedlings reached approximately 15 cm in height, using 0.50 m spacing between plants in single rows. Irrigation was provided daily. Cultural practices included vertical staking (“V” system), pruning, weeding, topdressing fertilization, and pest and disease management.
Herbicide application followed the same procedures as in the greenhouse. Visual phytotoxicity (0–100%) was evaluated at 7 and 14 DAA, plant height at 7, 14, 21, and 28 DAA, and fruit yield was recorded. Height was measured with a 2-m measuring tape on the four central plants. Fruits were harvested at ripening stage 4 (yellow-green calyx and orange skin), as described by (Santana et al., 2020), placed in plastic boxes, and weighed using a semi-analytical balance (Shimadzu UX2200H, 0.01 g precision).
Dose-response curve for pre-emergence herbicides in the field
A randomized complete block design with split-split plots and four replications was used. Biotypes 1 and 2 were assigned to the main plots. Pre-emergence herbicides imazaquin and S-metolachlor were applied at seven rates (0, 25, 50, 75, 100, 150, and 200% of 140 g ha⁻¹ and 800 g ha⁻¹, respectively), and a hand-weeded control was included, totaling 112 experimental units (4 × 1 m). All cultural practices were identical to those used in the previous field experiment. Spraying was carried out using flat-fan nozzles (110.02) spaced 0.50 m apart on a 2-m boom, delivering 200 L ha⁻¹. Immediately after application, overhead sprinklers applied 10 mm of irrigation to incorporate the herbicides.
The following variables were analyzed: phytotoxicity at 30 DAA, plant height at 14, 21, 35, and 50 DAA, weed incidence and control, and yield. Weed incidence was measured using a 1 m² quadrant at the exact location in each plot. Fruits were harvested under the same conditions as in the postemergence herbicide field experiment.
Phenolic compound analysis
After harvest, fruits were cleaned and analyzed at the Analytical Experimentation and Research Center (NEEA) of IFRS – Sertão Campus. Three fruits per treatment were randomly selected to determine soluble solids (°Brix). For aqueous extraction, fruits were macerated and mixed with distilled water (1:3 w/w) and left in an ultrasonic bath for 20 minutes without heating. The extract was filtered through gauze and re-extracted with fresh water. The same procedure was followed for ethanolic extraction, using 95% ethanol (1:3 w/w) instead of water. All extracts were refrigerated and protected from light.
Total phenolic content was determined using the Folin-Ciocalteu method (Pérez, Dominguez-López, & Lamuela-Raventós, 2023), with absorbance measured at 760 nm. An aliquot of 0.5 mL of extract was mixed with 2.5 mL of 10% Folin reagent and 2.0 mL of 7.5% sodium carbonate. The mixture was incubated for 5 minutes at 50 °C in a water bath. Using a standard curve, results were expressed as gallic acid equivalents (GAE). Analyses were performed in duplicate.
Statistical analysis
Data normality was verified using the Shapiro-Wilk test. When necessary, square root or square root of (x + 1) transformations were applied to zero-inflated data. Data were subjected to analysis of variance (ANOVA) using R software (R Core Team, 2025), and means were compared using Tukey’s test (p ≤ 0.05). Regression curves were fitted with a 95% confidence interval for dose-response experiments using SigmaPlot software v. 16.0 (Grafiti, 2024).
3. Results and discussion
Effect of post-emergence herbicides on Physalis peruviana
No significant interaction was observed between biotype × herbicide (p = 0.35) nor between biotypes 1 and 2 (p = 0.23). Thus, results are presented as the mean of both biotypes. Among the tested herbicides, chlorimuron caused the greatest reduction in plant height, significantly differing from other treatments (Figure 1). Fomesafen and atrazine + simazine also reduced plant growth at 14 DAA (Figure 1C), maintaining lower height values similar to chlorimuron at 21 DAA (Figure 1D). In contrast, ACCase-inhibiting herbicides-quizalofop, cletho-dim, fluazifop, and clodinafop - did not differ from the untreated control, indicating high selectivity to the crop.
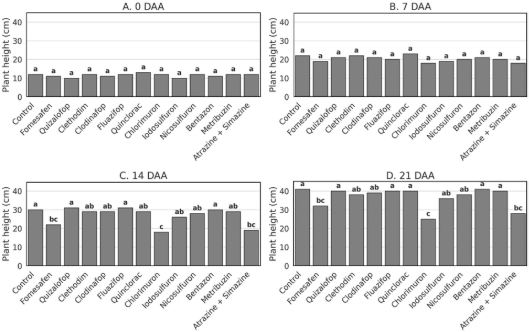
Figure 1. Plant height of Physalis peruviana at 0, 7, 14, and 21 days after postemergence herbicide application under greenhouse conditions. Bars represent treatment means followed by the same letter, which do not differ statistically according to Tukey’s test (p ≤ 0.05).
In the first year of the field trial, no differences among herbicide treatments were detected at 7 DAA (Figure 2B). Similar to the greenhouse experiment, chlorimuron stood out for significantly reducing plant height, followed by iodosulfuron and nicosulfuron, which also caused substantial reductions. Conversely, quizalofop, clethodim, clodinafop, and fluazifop maintained plant height similar to the untreated control, confirming their selectivity. Additionally, metribuzin and bentazon showed favorable performance, with growth comparable to or exceeding the control treatment, especially at 21 DAA (Figure 2D).
In the second year of the field experiment, marked differences among treatments became evident from 7 DAA onward (Figure 3D), particularly with fomesafen, which significantly reduced plant height. This response intensified at 14 and 21 DAA (Figures 3C and 3D). Similarly, chlorimuron, iodosulfuron, and, to a lesser extent, atrazine + simazine, again reduced growth, confirming the trend observed in the previous year. In contrast, quizalofop, clethodim, clodinafop, and fluazifop maintained height values similar to the control, confirming their consistent selectivity across both years. Bentazon and metribuzin also performed well, showing consistent results and reinforcing their potential selectivity for the crop.
The lack of differential response between physalis biotypes to postemergence herbicides may be attributed to the absence of physiological or biochemical variation affecting herbicide absorption, translocation, or metabolism (de Oliveira et al., 2025; Jones et al., 2024). Previous studies on P. peruviana suggest herbicide sensitivity can depend on the application stage and environmental conditions, such as moisture and temperature, influencing herbicide efficacy.
Among the herbicides tested, chlorimuron exhibited high phytotoxicity, significantly reducing plant height under greenhouse and field conditions. Chlorimuron inhibits acetolactate synthase (ALS), rapidly affecting cell division and meristematic tissue elongation in sensitive species (Yu & Powles, 2014). On the other hand, ACCase-inhibiting herbicides (quizalofop, clethodim, clodinafop, and fluazifop) showed high selectivity. This is consistent with the known structural differences in acetyl-CoA carboxylase between eudicots and monocots, which confer natural tolerance to broadleaf species (Jin, 2025).
Bentazon and metribuzin maintained favorable selectivity patterns, emerging as promising alternatives for weed management in physalis. Metribuzin selectivity may be linked to the crop’s ability to rapidly metabolize the herbicide, a critical factor distinguishing tolerant from susceptible species (Kudsk & Streibig, 2003). The variation in fomesafen response between years, with greater phytotoxicity in the second year, likely reflects the influence of environmental factors on herbicide uptake and translocation (Cobb & Reade, 2011), underscoring the importance of multi-year evaluations for herbicide recommendations.
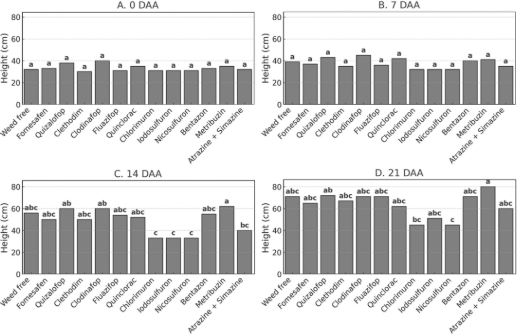
Figure 2. Plant height of P. peruviana at 0, 7, 14, and 21 days after postemergence herbicide application in the first year of the field experiment. Bars represent treatment means followed by the same letter, which do not differ statistically according to Tukey’s test (p ≤ 0.05).
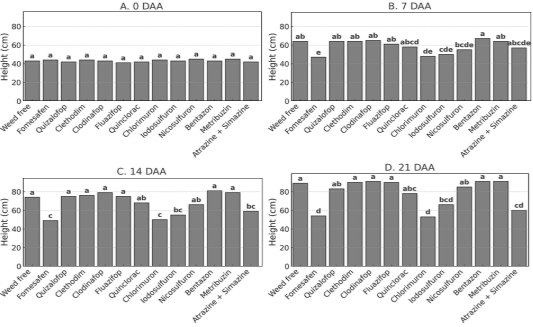
Figure 3. Plant height of P. peruviana at 0, 7, 14, and 21 days after postemergence herbicide application in the second year of the field experiment. Bars represent treatment means followed by the same letter, which do not differ statistically according to Tukey’s test (p ≤ 0.05).
Regarding phytotoxicity assessments in the first year, fomesafen and atrazine + simazine caused injury levels exceeding 30% at 7 DAA (Figure 4A). At 14 DAA (Figure 4B), the atrazine + simazine mixture produced the highest injury scores, followed by fomesafen and chlorimuron, indicating progressive damage over time. At 21 DAA (Figure 4C), injury symptoms intensified for iodosulfuron, chlorimuron, and atrazine + simazine, all-surpassing 60%, while most other herbicides maintained low injury levels. By 28 DAA (Figure 4D), fomesafen, iodosulfuron, chlorimuron, and atrazine + simazine continued to exhibit high levels of crop injury. In contrast, ACCase inhibitors (quizalofop, clethodim, clodinafop, and fluazifop) caused no visible injury at any evaluation time, reinforcing their selectivity to P. peruviana.
In the second year, fomesafen and atrazine + simazine again caused phytotoxicity above 30% at 7 DAA (Figure 5A), while chlorimuron and iodosulfuron induced intermediate visual injury. At 14 DAA (Figure 5B), atrazine + simazine remained the most injurious treatment, followed by fomesafen and chlorimuron, showing similar behavior to the previous year. At 21 DAA (Figure 5C), injury intensified in plots treated with iodosulfuron, chlorimuron, and atrazine + simazine, all exceeding 60%. These treatments maintained high injury levels at 28 DAA (Figure 5D), suggesting prolonged action. The ACCase-inhibiting herbicides again caused no visible phytotoxicity, confirming their safety to the crop.
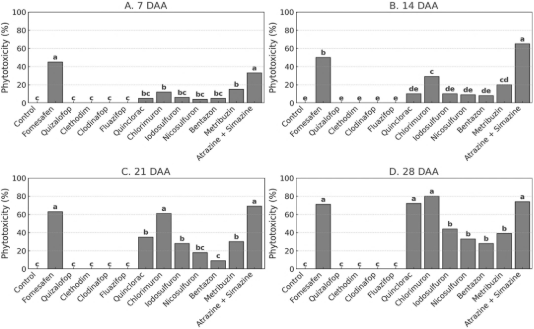
Figure 4. Phytotoxicity of Physalis peruviana at 7, 14, 21, and 28 days after postemergence herbicide application in the first year of the field experiment. Bars represent treatment means followed by the same letter, which do not differ statistically according to Tukey’s test (p ≤ 0.05).
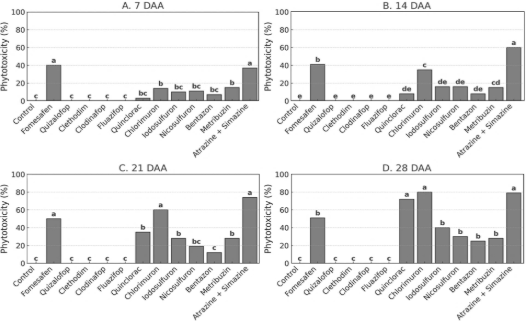
Figure 5. Phytotoxicity of P. peruviana at 7, 14, 21, and 28 days after postemergence herbicide application in the second year of the field experiment. Bars represent treatment means followed by the same letter, which do not differ statistically according to Tukey’s test (p ≤ 0.05).
The results of this study indicate that Physalis peruviana exhibits variable sensitivity to postemergence herbicides, being more susceptible to PPO inhibitors (fomesafen), ALS inhibitors (iodosulfuron and chlorimuron), and photosystem II inhibitors (atrazine + simazine). These findings are consistent with previous reports involving species of the Physalis genus and other Solanaceae crops. For instance, Physalis angulata (Dhaouadi et al., 2022) showed significant injury following postemergence fomesafen application, with marked reductions in shoot dry mass and leaf area (Chiconi et al., 2022). Similarly, atrazine applications in P. peruviana caused notable phytotoxic effects, negatively impacting crop development (Khodadadi et al., 2023).
In tomato (Solanum lycopersicum), ALS-inhibiting herbicides such as chlorimuron have been associated with cumulative stress and yield reduction (McNaughton, 2013). Conversely, clethodim and other ACCase inhibitors are recognized for their selectivity in broadleaf crops, including Solanaceae species, due to the structural specificity of the target enzyme in monocots (Kukorelli et al., 2013).
Yield data for Physalis peruviana over two growing seasons (Figure 6) revealed high variability with no statistically significant differences. This variability is likely associated with this crop's lack of genetically stabilized cultivars. In the first season (Figure 6A), the highest yields were recorded in the untreated control and plots treated with bentazon, clethodim, quizalofop, and fluazifop, all showing median values above 200 g plant⁻¹. Conversely, fomesafen, chlorimuron, iodosulfuron, quinclorac, and atrazine + simazine markedly reduced fruit production, with mean values below 100 g plant⁻¹.
A similar pattern was observed in the second season (Figure 6B). Once again, bentazon achieved the widest yield range with a median comparable to the control, supporting its selectivity and potential use in physalis. The grass herbicides quizalofop, clethodim, and fluazifop also maintained high yield values, reinforcing their selective profile. On the other hand, fomesafen, chlorimuron, and quinclorac resulted in severely reduced yields, with mean values close to zero, indicating high levels of crop injury.
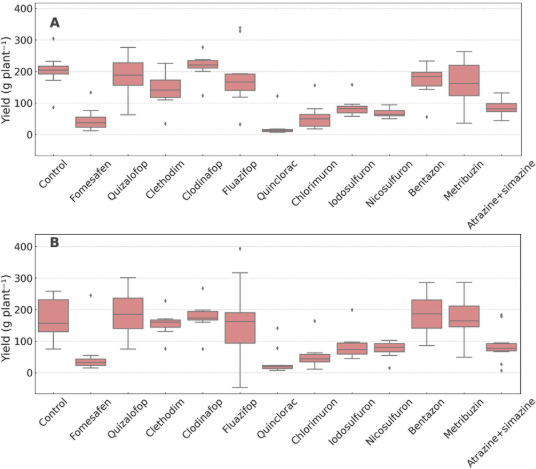
Figure 6. Fruit yield of Physalis peruviana (g plant⁻¹) under postemergence herbicide treatments in two growing seasons: (A) first year and (B) second year.
The yield of P. peruviana is strongly influenced by factors such as nutrient management, plant spacing, environmental conditions, and herbicide use. Previous studies have shown that herbicides like linuron can cause high mortality and drastic reductions in fruit production, highlighting the importance of selectivity (Khodadadi et al., 2023). Under favorable conditions, yields between 5 and 7 t ha⁻¹ are expected in the first season, reaching up to 15 t ha⁻¹ in the second (Muniz et al., 2015). These findings reinforce the need for integrated and selective weed control strategies to ensure productive success in P. peruviana cultivation.
Effect of pre-emergence herbicides on Physalis peruviana
The height of P. peruviana plants at 50 days after application (DAA) was influenced by increasing doses of S-metolachlor and imazaquin (Figure 7). S-metolachlor did not significantly reduce height at any dose or biotype, with average plant heights exceeding 67 cm even at the highest dose (1600 g ha⁻¹). In contrast, imazaquin caused dose-dependent reductions in plant height, with the most pronounced effect in biotype 1. These results indicate that imazaquin, compared to S-metolachlor, exerts a more significant impact on early plant development, affecting shoot growth in both biotypes.
S-metolachlor, a selective pre-emergence herbicide used for controlling annual grasses and some small-seeded broadleaf species, did not significantly affect plant height in either biotype, suggesting tolerance by P. peruviana (Rosenthal et al., 2006). It has demonstrated effective weed control with minimal impact on broadleaf crops (Besançon et al., 2020). In contrast, imazaquin (an ALS-inhibiting imidazolinone) significantly reduced plant height, especially in biotype 1. ALS inhibitors disrupt the synthesis of essential amino acids, leading to stunted growth and chlorosis in new tissues (Velasquez et al., 2024). This differential response may stem from genetic variation between the biotypes, influencing sensitivity to imazaquin. Similar reductions in biomass and height due to ALS inhibitors have been reported in sensitive species (Dai et al., 2025).
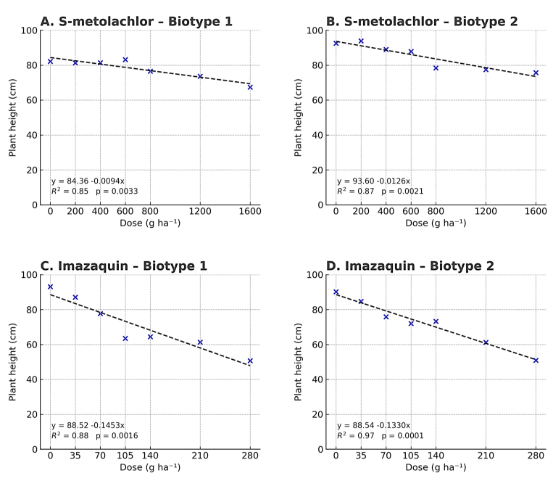
Figure 7. Plant height at 50 days after application as a function of herbicide dose in two Physalis peruviana biotypes.
Linear, quadratic, and nonlinear regression analyses were applied to yield data, but no significant models were detected (p > 0.05), indicating no consistent relationship between herbicide dose and productivity. ANOVA results were also not significant. In some cases, such as biotype 1 treated with imazaquin, high yield variation was observed (49.87 to 154.67 g plant⁻¹ at 35 g ha⁻¹). Similarly, S-metolachlor at 200 g ha⁻¹ resulted in yields ranging from 97.79 to 168.51 g plant⁻¹ in biotype 2. This variability likely results from the genetic instability of the crop, leading to agronomically heterogeneous plant responses. Thus, boxplots were considered more appropriate for interpreting herbicide effects across biotypes.
Increasing doses of S-metolachlor and imazaquin also reduced weed density in treated plots (Figure 9). The predominant weed species were Galinsoga parviflora and Portulaca oleracea. A clear dose-response relationship was observed for S-metolachlor, with a progressive decline in weed density as herbicide rates increased, especially in Figures 9A and 9B. Imazaquin also reduced weed emergence, although with less consistent effects across plots (Figures 9C and 9D). These variations were attributed to differences in the floristic composition of weed communities rather than biotype sensitivity. Hence, the results highlight the importance of the herbicide spectrum when selecting products for pre-emergence weed management.
The predominant weed species in the experimental areas were Galinsoga parviflora and Portulaca oleracea, both known for their high emergence potential and rapid growth, making them particularly problematic in vegetable cropping systems and areas with reduced soil cover. These species emerge rapidly after soil disturbance, justifying pre-emergence herbicides as a management strategy. G. parviflora (Asteraceae) exhibits fast growth, can produce viable seeds within days of emergence, and is highly sensitive to very long-chain fatty acid synthesis inhibitors such as S-metolachlor (Kashe et al., 2020). In contrast, P. oleracea (Portulacaceae) is tolerant to several herbicides and maintains a persistent soil seedbank, requiring consistent control measures to prevent re-infestation (Raja et al., 2024). The experimental results confirm the partial effectiveness of imazaquin against these species but highlight the superior performance of S-metolachlor as a more robust tool for pre-emergence control of these hard-to-manage weeds.
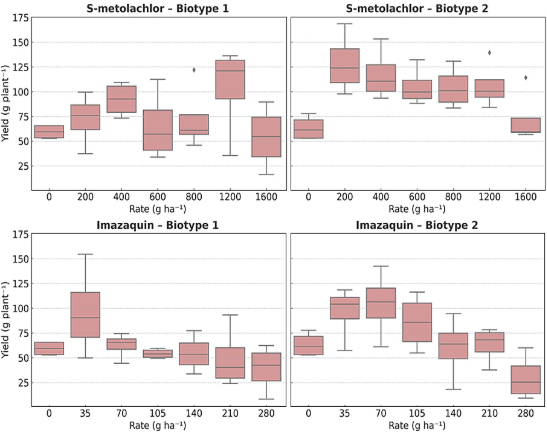
Figure 8. Boxplots of plant yield in response to increasing doses of S-metolachlor and imazaquin in two Physalis peruviana biotypes.
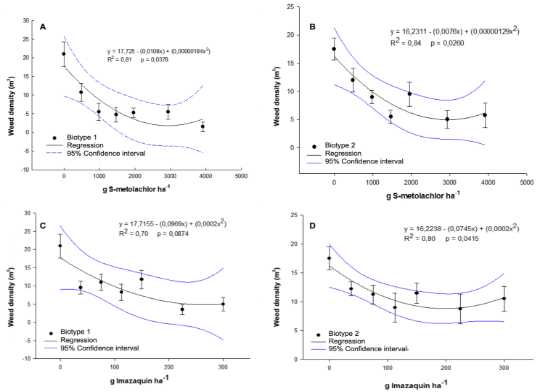
Figure 9. Dose-response curves for S-metolachlor and imazaquin in two P. peruviana biotypes.
Effect of pre-emergence herbicides and weed presence on total phenolic content in Physalis peruviana
A significant difference in total phenolic content was detected between extraction methods (p < 0.001, t-test), so results are presented separately for each method. The overall mean for the aqueous extraction was 135.5 µg gallic acid equivalents (GAE) per g of sample, while the ethanolic method averaged 94.7 µg GAE g⁻¹. ANOVA for the aqueous method indicated significant effects of weed control methods and their interaction with biotypes.
In biotype 1, no differences were observed among weed control methods. In biotype 2, however, plants grown under weed-free conditions had the highest total phenolic levels, reaching 197.8 µg GAE g⁻¹ (Table 2). These results support the hypothesis that high weed pressure and herbicide-induced stress reduce phenolic content in P. peruviana fruits. In untreated plots, weed density reached 19.25 plants m⁻², while herbicide treatments led to crop injury levels of 43% (imazaquin) and 38% (S-metolachlor) (Table 3). Across both biotypes, biotype 2 consistently produced higher phenolic levels, suggesting genotypic differences as both were grown under the same conditions.
Table 3
Weed density (plants m⁻²) and phytotoxicity levels in P. peruviana as affected by weed control method
Treatments | Weed density (m-2) | Toxicity |
Control | 19.25 | a | 0 | b |
Weed free | 0.00 | c | 0 | b |
Imazaquin | 7.75 | b | 43 | a |
S-metolachlor | 3.62 | b | 38 | a |
¹ Means followed by different letters differ according to Tukey’s test (p ≤ 0.05).
Table 2
Total phenolic content (µg gallic acid equivalents g⁻¹ fresh weight) in P. peruviana fruits as affected by biotype and weed control method using aqueous and ethanolic extraction
Aqueous extraction method |
Biotype | Weed control methods |
Control | Weed free | Imazaquin | S-metolachlor |
Biotype 1 | 132.9 aA¹ | 144.1 bA | 109.3 aA | 143.4 aA |
Biotype 2 | 130.8 aB¹ | 197.8 aA | 98.3 aB | 127.0 aB |
Ethanolic extraction method |
Biotypes | Weed control methods |
Control | Weed free | Imazaquin | S-metolachlor |
Biotype 1 + 2 | 64.2 B | 112.0 A | 98.2 AB | 104.4 AB |
¹ Means followed by different letters differ according to Tukey’s test (p ≤ 0.05), lowercase letters within columns, and uppercase letters across rows.
ANOVA showed significant effects of both biotype and weed control for the ethanolic method. Biotype 1 had lower phenolic content (77.6 µg GAE g⁻¹) than biotype 2 (111.8 µg GAE g⁻¹). Similar to the aqueous method, this difference was attributed to genotypic variability. Comparing weed control methods, it is evident that high weed densities reduce the synthesis and accumulation of phenolic compounds (Table 2). This reduction likely occurs due to resource competition, which limits photosynthesis and other metabolic processes. Plants may increase phenolic production as a defense response under moderate stress, such as low weed pressure or minimal herbicide injury. Indeed, applying herbicides like alachlor and rimsulfuron increased phenolic compound production in maize and soybeans. The growing conditions, and the herbicides, must be carefully chosen because they affect the phytochemical components and especially the metabolism of polyphenols (Yari et al., 2025).
4. Conclusions
Physalis peruviana was sensitive to chlorimuron, fomesafen, iodosulfuron, and the atrazine + simazine mixture when applied post-emergence. ACCase-inhibiting herbicides were selective to the crops and represent promising options for weed management. Bentazon and metribuzin showed low phytotoxicity, while imazaquin reduced plant growth, unlike S-metolachlor, which did not affect plant height. The presence of weeds and herbicide applications reduced the total phenolic content in the fruits, potentially compromising their functional quality.
These findings are relevant for growers, agricultural consultants, and researchers interested in the chemical management of P. peruviana, especially in systems where selective herbicide use is considered. The results provide guidance for selecting safe herbicide molecules that enable effective weed control without harming the crop’s phytochemical potential.
Future studies should evaluate the efficacy of these molecules at different crop stages and under various soil and climate conditions, as well as assess potential residual effects on soil microbiota and the nutritional composition of the fruits over time.
Acknowledgements
The authors would like to express their gratitude to the Brazilian federal agencies: Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). They also acknowledge the support of artificial intelligence tools in proofreading and grammar-checking this manuscript to make the text more fluid and formal (Grammarly, 2025; OpenAI, 2025).
Authors’ contributions
Conceptualization of the manuscript and development of the methodology: A.L.N.G., D.B., and W.L.P.; data collection and curation: S.G.S., R.C., and R.A.P.; data analysis: all authors; data interpretation: all authors funding acquisition and resources: A.L.N.G., and W.L.P.; project administration: A.L.N.G., D.B., and W.L.P.; supervision: A.L.N.G.; writing the original draft of the manuscript: S.G.S., and A.L.N.G.; writing, review and editing: all authors. All authors read and agreed to the published version of the manuscript.
ORCID
A. L. N. Gabardo https://orcid.org/0000-0002-4789-0253
D. Bilibio https://orcid.org/0000-0002-3393-7049
W. L. Priamo https://orcid.org/0000-0001-9983-9583
S. G. Sossmeier https://orcid.org/0000-0002-1461-2994
R. A. Polito https://orcid.org/0000-0001-9452-8137
R. Cinelli https://orcid.org/0000-0003-4659-6762
References
Besançon, T. E., Wasacz, M. H., & Carr, B. L. (2020). Weed control and crop tolerance with S-metolachlor in seeded summer squash and cucumber. Weed Technology, 34(6), 849-856. https://doi.org/10.1017/wet.2020.72
Chiconi, L. A., Bacha, A. L., Braga, A. F., Carrega, W. C., Nepomuceno, M. P., & Alves, P. L. d. C. A. (2022). Selectivity of herbicides isolated and/or with the addition of adjuvants for <i>Physalis angulata</i> crop. Horticultura Brasileira, 40. https://doi.org/10.1590/s0102-0536-20220202
Cobb, A. H., & Reade, J. P. H. (2011). Herbicides and Plant Physiology: Wiley.
Coleman, M. J., Kristiansen, P., Sindel, B. M., & Fyfe, C. (2024). Imperatives for integrated weed management in vegetable production: Evaluating research and adoption. Weed Biology and Management, 24(1), 3-14. https://doi.org/10.1111/wbm.12285
Dai, S. Z., Wang, Y. W., Yook, M. J., Wu, H. Z., Chen, M., & Zhang, C. J. (2025). Screening of Pre- and Post-Emergence Herbicides for Weed Control in Camelina sativa (L.) Crantz. Agronomy-Basel, 15(3). https://doi.org/10.3390/agronomy15030640
de Oliveira, C., Mathioni, S. M., Riaño, A. D., Camargo, E. R., Dornelles, S. H. B., Lemes, L. N., . . . Avila, L. A. (2025). First report of barnyardgrass resistant to glyphosate in Brazil. Advances in Weed Science, 43. https://doi.org/10.51694/AdvWeedSci/2025;43:00010
Dhaouadi, F., Sellaoui, L., Taamalli, S., Louis, F., El Bakali, A., Badawi, M., . . . Rtimi, S. (2022). Enhanced adsorption of ketoprofen and 2,4-dichlorophenoxyactic acid on Physalis peruviana fruit residue functionalized with H2SO4: Adsorption properties and statistical physics modeling. Chemical Engineering Journal, 445. https://doi.org/10.1016/j.cej.2022.136773
Dittmar, P., Monks, D., Jennings, K., & Booker, F. (2012). Tolerance of Tomato to Herbicides Applied through Drip Irrigation. Weed Technology, 26, 684-690. https://doi.org/10.2307/23358271
Gazola, T., Gomes, D. M., Belapart, D., Dias, M. F., Carbonari, C. A., & Velini, E. D. (2021). Selectivity and residual weed control of pre-emergent herbicides in soybean crop. Revista Ceres, 68. https://doi.org/10.1590/0034-737X202168030008
Gnanasekaran, P. (2022). Effect of Post-Emergence Herbicides on Control of Weeds in Tomato During Kharif Season. International Journal of Farm Sciences, 12, 134-138. https://doi.org/10.5958/2250-0499.2022.00088.X
Grafiti. (2024). Sigmaplot. Palo Alto: Grafiti LLC.
Grammarly. (2025). Grammarly desktop. San Francisco. Retrieved from https://www.grammarly.com
Guiné, R. P. F., Gonçalves, F. J. A., Oliveira, S. F., & Correia, P. M. R. (2020). Evaluation of Phenolic Compounds, Antioxidant Activity and Bioaccessibility in Physalis peruviana L. International Journal of Fruit Science, 20(sup2), S470-S490. https://doi.org/10.1080/15538362.2020.1741056
Jin, Y. (2025). Validation of assay for measuring acetyl-coenzyme a carboxylase activity in grasses using malachite green. Analytical Biochemistry, 697. https://doi.org/10.1016/j.ab.2024.115723
Jones, E. A. L., Contreras, D. J., Argueta, R. J., Bradshaw, C., Leon, R. G., & Everman, W. J. (2024). Control of ALS- and PPO-inhibiting herbicide-resistant redroot pigweed (Amaranthus retroflexus) populations with common postemergence herbicides. Advances in Weed Science, 42. https://doi.org/10.51694/AdvWeedSci/2024;42:00039
Kashe, K., Ketumile, D., Kristiansen, P., Mahilo, C., & Moroke, T. (2020). Evaluation of pre-emergence herbicides for weed control in maize. Welwitschia International Journal of Agricultural Sciences, 2, 5-18. https://doi.org/10.32642/wijas.v2i.1437
Khodadadi, V., Yousefi, A. R., Shahbazi, S., Heydari, M., Kanatas, P., Tataridas, A., & Travlos, I. (2023). Evaluation of herbicides for selective weed control in cutleaf groundcherry (Physalis angulata L.). Crop Protection, 169. https://doi.org/10.1016/j.cropro.2023.106243
Kudsk, P., & Streibig, J. C. (2003). Herbicides – a two-edged sword. Weed Research, 43(2), 90-102. /https://doi.org/10.1046/j.1365-3180.2003.00328.x
Kukorelli, G., Reisinger, P. W. M., & Pinke, G. (2013). ACCase inhibitor herbicides – selectivity, weed resistance and fitness cost: a review. International Journal of Pest Management, 59, 165 - 173. https://doi.org/10.1080/09670874.2013.821212
McNaughton, K. E. (2013). Cumulative herbicide stress on processing tomato (Solanum lycopersicum L.). University of Guelph,
Muniz, J., Molina, A. R., & Muniz, J. (2015). Physalis: Panorama produtivo e econômico no Brasil. Horticultura Brasileira, 33. https://doi.org/10.1590/S0102-053620150000200023
Nosratti, I., Sabeti, P., Chaghamirzaee, G., & Heidari, H. (2020). Weed problems, challenges, and opportunities in Iran. Crop Protection, 134. https://doi.org/10.1016/j.cropro.2017.10.007
OpenAI. (2025). ChatGPT. San Francisco: OpenAi. Retrieved from https://chat.openai.com/
Pérez, M., Dominguez-López, I., & Lamuela-Raventós, R. M. (2023). The Chemistry Behind the Folin-Ciocalteu Method for the Estimation of (Poly)phenol Content in Food: Total Phenolic Intake in a Mediterranean Dietary Pattern. J Agric Food Chem, 71(46), 17543-17553. https://doi.org/10.1021/acs.jafc.3c04022
R Development Core Team. (2025). A language and environment for statistical computing. Viena: R Foundation for Statistical Computing. Retrieved from https://www.r-project.org
Raja, T., Devarajan, Y., Jayasankar, P., Singh, D., Subbiah, G., & Logesh, K. (2024). Characterization and sustainable applications of galinsoga parviflora natural fibers: A pathway to eco-friendly material development. Results in Engineering, 24. https://doi.org/10.1016/j.rineng.2024.103601
Rosenthal, M. D. A., Procópio, S. O., Pinto, J. J. O., Júnior, E. A. J., Peres, W. B., Manica, R., . . . Franzini, W. (2006). S-metholachlor toxicity in maize plants originated from seeds with different sizes and shapes. 24(2), 319-327. https://doi.org/10.1590/s0100-83582006000200015
Santana, A. S., Giacobbo, C. L., Prado, J. d., Uberti, A., Louis, B., & Alberto, C. M. (2020). Fenologia e qualidade de frutos de acessos de Physalis spp. Agrarian, 13(47), 1-8. https://doi.org/10.30612/agrarian.v13i47.8687
Santos, H. G. d., Jacomine, P. K. T., Anjos, L. H. C. d., Oliveira, V. A. d., Lumbreras, J. F., Coelho, M. R., . . . Cunha, T. J. F. (2018). Sistema Brasileiro de Classificação de Solos (5 ed.). Brasiília: Embrapa.
Uljol, L. H. O., Bianco, S., Filho, A. B. C., Bianco, M. S., & Carvalho, L. B. (2018). Weed Interference on Productivity of Bell Pepper Crops. Planta Daninha, 36. https://doi.org/10.1590/S0100-83582018360100046
Velasquez, J. C., Hoyos, V., Roma-Burgos, N., & Plaza, G. (2024). Weedy rice resistance to imidazolinone herbicides and control with glyphosate. Advances in Weed Science, 42. https://doi.org/10.51694/AdvWeedSci/2024;42:00035
Yari, P., Alirezalu, A., & Khalili, S. (2025). A comparative study of chemical composition, phenolic compound profile and antioxidant activity of wild grown, field and greenhouse cultivated Physalis (P. alkekengi and P. peruviana). Food Production, Processing and Nutrition, 7(1), 19. https://doi.org/10.1186/s43014-024-00287-9
Yu, Q., & Powles, S. B. (2014). Metabolism-based herbicide resistance and cross-resistance in crop weeds: a threat to herbicide sustainability and global crop production. Plant Physiology, 166(3), 1106-1118. https://doi.org/10.1104/pp.114.242750