RESEARCH ARTICLE
Antixenosis of different maize genotypes in storage affects feeding preferences and oviposition of Sitophilus zeamais Moth
Rodrigues Agostinho Marcos1 *; Filipe Garcia Holtz2; Moisés Moura de Oliveira Ramos2
Josimar Aleixo da Silva2; Maria Lúcia Ferreira Simeone3; Hugo Bolsoni Zago2
Fábio Luiz De Oliveira2; Leandro Pin Dalvi2
1 Agricultural Research Institute of Mozambique, Northeast Zonal Center, Nampula, Mozambique.
2 Federal University of Espírito Santo. Alegre Campus, ES, Brazil
3 Embrapa Milho e Sorgo, MG 424, km 45, 35701-970, Sete Lagoas, MG, Brazil.
* Corresponding author: rodamarcos0@gmail.com (R. A. Marcos).
Received: 12 December 2024. Accepted: 18 May 2025. Published: 3 June 2025.
Abstract
During corn storage, significant losses occur due to pest attacks, especially the weevil, Sitophilus zeamais Motschulsky. This study aimed to evaluate the feeding preference of Sitophilus zeamais on seeds of stored corn genotypes. The tests were carried out in the laboratory of the Center for Scientific and Technological Development in Pest and Disease Management (NUDEMAFI) at the Center for Agricultural Sciences and Engineering of the Federal University of Espírito Santo (UFES) in Alegre, in an air-conditioned room with a maximum temperature of 26.4 and a minimum of 26.2 ºC (± 2 ºC) and humidity between 70% and 75%. A host preference test with free choice was performed on insects from Nudemafi breeding, aged 5 to 10 days, in six (6) arenas with six (6) replicates using a completely randomized design (CRD). After 96 hours, the following were evaluated for each genotype: number of insects attracted, insect weight, number of infested seeds, percentage of seed loss, and 60 days after infestation, the percentage of emerged insects, physical and bromatological composition were determined. The results showed significant differences in the preference of Sitophilus zeamais adults in relation to the maize genotypes analyzed; the lowest food preference was observed in the Purple genotype (4.1%), followed by AG1051, which obtained 8.3% respectively. The genotypes presented antixenosis due to the effect of the nutritional properties and physical hardness of the seed.
Keywords: antixenosis; maize; post-harvest; Sitophilus zeamais.
DOI: https://doi.org/10.17268/sci.agropecu.2025.031
Cite this article:
Marcos, R. A., Holtz, F. G., Ramos, M. M. O., da Silva, J. A., Simeone, M. L. F., Zago, H. B., De Oliveira, F. L., & Dalvi, L. P. (2025). Antixenosis of different maize genotypes in storage affects feeding preferences and oviposition of Sitophilus zeamais Moth. Scientia Agropecuaria, 16(3), 409-416.
1. Introduction
Seeds are the main productive input in agricultural activity, as they are the repository of agronomic characteristics. Among the limiting factors in the maize production chain, attacks by insect pests can cause losses to the crop from the field to storage (Cruz, 2008; Conceição et al., 2024). In this case, the corn weevil, Sitophilus zeamais Mots.1855 (Coleoptera: Curculionidea), attacks grains from the field to the warehouse, has a high biotic potential and can survive at great depths in the grain mass (Gallo et al., 2002; Frazão et al., 2018). Adults pierce the grain to lay eggs, and larvae feed on the grain endosperm until they transform into pupae. When they become adults, they pierce the grain and emerge into the environment. Females can lay up to 250 eggs during their reproductive life. The life cycle depends on the temperature and varies between 30 and 113 days. In temperate zones, there are two to three generations per year (García-Lara et al., 2007).
The main curative method for controlling corn weevils is the use of fuming chemicals, such as aluminum phosphide (Fazolin et al., 2010) and magnesium phosphide. Given these difficulties in control, mainly due to the availability of new molecules for the chemical control of these insect pests, there is the possibility of using resistant varieties (Nwosu, 2016). Research has reported accessions of corn genotypes resistant to Sitophilus zeamais such as Ikenne 83-TZSR-W-1 (open pollination) and hybrid 8329-15 (Kim & Kossou et al., 2003), and the varieties ZM421 and ZM521 showed potential for suppressing Sitophilus zeamais (Muzemu & Goto, 2013; Alves & Poltronieri, 2024).
Given these considerations, there is a need to conduct research to identify maize genotypes that present resistance mechanisms, either by non-preference for feeding and oviposition or by antixenosis. Therefore, this study aimed to evaluate the host preference of Sitophilus zeamais in seeds of stored maize genotypes.
2. Methodology
To evaluate Antisenosis (non-host preference), the tests were performed through the free choice test in the laboratory of the Center for Scientific and Technological Development in Pest and Disease Management (NUDEMAFI) at the Center for Agricultural Sciences and Engineering of the Federal University of Espírito Santo (UFES) in Alegre, Espírito Santo, Brazil, located at the coordinates latitude 20° 45' 49" S and longitude 41° 31' 58" W.
820 g of standardized seeds of six (6) maize genotypes were used (Figure 1), two (2) commercial hybrids (AG1051 and Glyfos RR) and four (4) varieties (Roxo, Vermelho, Palha roxa and Branco corn.

Figure 1. Maize genotypes used in the S. zeamais non-preference test. G1: Branco; G2: Glyfos RR; G3: AG1051; G4: Roxo; G6: Palha roxa; G5: Vermelho.
Obtaining and characterizing seeds of corn genotypes
The seeds used in the test came from the genotype collection of the plant analysis laboratory of the Department of Agronomy of the Center of Agricultural Sciences of UFES. The corn seeds were kept in plastic bags at a temperature of 5 °C for 15 days to eliminate the possibility of latent infestation by grain pests. After this period, the moisture content of the seeds (Table 1) was determined using the greenhouse method at a temperature of 105 ± 3 °C for 24 hours (Brasil, 2009).
Table 1
Moisture content (%), Weight (g), grain color and seed morphology of maize genotypes
Genotypes | Humidity (%) | Weight of 1000 Se | Grain color | Grain shape |
Branco | 10.2 | 287.7 | White | Toothed |
Glyfos RR | 10.3 | 207.3 | Yellow | Semi-hard |
AG1051 | 10.3 | 283.5 | Yellow | Toothed |
Roxo | 10.4 | 300.7 | Colors | Hard |
Vermelho | 10.5 | 213.5 | Colors | Hard |
Palha roxa | 10.3 | 248.3 | Yellow | Toothed |
Greenhouse method | 105 ± 3 °C /24 h (g) | - | - |
Se: seeds; (g): grams.
Obtaining and rearing the insects used in the host non-preference test
The 140 unsexed adult insects (F1) used in this experiment were obtained from pure populations from the entomology laboratory of NUDEMAFI, fed with dent Crioulo yellow corn sold in Alegre - ES. For this purpose, 6 wide-mouthed glass containers with a capacity of 2 liters containing 200 g of white corn grains and 50 unsexed adult insects were used to lay eggs. The containers were closed with voile fabric, secured around the mouth of the container with elastic gum, which prevented the insects from escaping and allowed aeration (Figure 2a).
The containers containing the insects were kept in a climate-controlled room with a maximum temperature of 26.4 and a minimum of 26.2 ºC (± 2 ºC) with 70% to 75% humidity and a 12-hour photophase. After a 10-day period in the containers, the adult insects were removed from the containers and discarded. Then, the containers containing corn grains with the eggs were kept under the same climatic conditions until the emergence of the adult insects of the F1 generation, which were later used for the test, using the method recommended by (Rossetto, 1972).
Using insects from the breeding, aged 5 to 10 days, the host non-preference test was carried out, conducted with a chance to choose in six (6) arenas with six (6) replicates using a completely randomized design. Each arena was composed of seven (7) transparent polyethylene containers with a capacity of 100 ml. Six (6) 10 cm plastic microtubes were interconnected in the central container, arranged equidistant from each other, allowing the adult insects to move freely (Figure 2b).
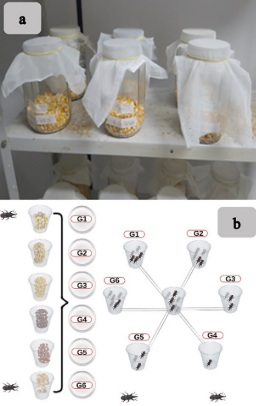
Figure 2. Insect rearing (2a) and host preference test arenas (2b). Maize and insect genotypes arenas: G1: Branco; G2: Glyfos RR; G3: AG1051; G4: Roxo; G6: Palha roxa; G5: Vermelho.
Host Antixenose (Non-Preference) Assay
In each of the six (6) containers, 20 g of corn seeds of each genotype were placed, randomly distributed, and in the central container, 20 adult insects of Sitophilus zeamais, unsexed and aged between 1 and 10 days, were released. After 96 h, the number of insects attracted was evaluated for each genotype, determined by Insects attracted (IA). At the same time, the adult insects were weighed using a 0.001 g analytical balance.
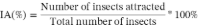
Through an infestation test, seeds with exit holes or damage caused by insects were analyzed, determining the number of infested seeds. At the same time, the percentage of infestation (%I) was determined using the following equation.

Seed losses (PDS) were also evaluated using the modified gravimetric method of Compton et al. (1998) based on the following equation:  . Where PSnd: weight of undamaged seeds; Pfa: final weight of the sample.
. Where PSnd: weight of undamaged seeds; Pfa: final weight of the sample.
After analysis, the uninfected seeds were left in hermetically sealed 100 ml containers for 60 days to evaluate the number of emerged F2 insects (% of progeny).
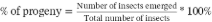 .
.
The infested seeds and adult insects were discarded, leaving only non-infested seeds for physical and centesimal hardness analysis. The physical hardness of the seed was determined using a D2240-15 durometer, based on seed penetration, using 6 replicates of 25 seeds. After this process, 15 g of seeds were crushed and ground in a TE 631- Tecnal- RPM 27000 type mill. After grinding, the samples were passed through a 20 mesh sieve with a granulometry of 0.5 mm and packaged in transparent polyethylene bottles and then taken to the Centesimal Composition Laboratory of Embrapa Milho e Sorgo.
Using near-infrared spectroscopy, analysis of the centesimal composition was performed using the NIRFlex solids N-500 equipment, model N-500, following the calibration methodology for corn and sorghum (Simeone et al., 2024) determining crude protein, ether extract, crude fiber and starch.
Statistical analysis
The data were subjected to analysis of variance, normality of residuals test (Shapiro-Wilk) and homo-geneity of variances (Bartlet), and the variables were compared by the F test at a 5% probability level. When significant, the genotypes were subjected to the Scott-Knott test to compare means. Through multivariate analysis, Pearson's correlation was performed for different characteristics of the genotypes in relation to the preference for the insect pest, where the Pearson correlation coefficient measures the strength and direction of the linear relationship between two variables with a variation from -1 to 1. Therefore, the absolute value of the correlation coefficient indicates the strength of the relationship between the variables analyzed in the research, with significant and strong relationships being grouped together as they are closer to 1 for different characteristics of the genotypes in relation to preference for the insect pest. All statistical analyses were performed using R software (R CORE TEAM, 2021).
3. Results and discussion
In the host non-preference bioassay performed by the free choice test, significant differences were found ap (≤ 0.05) in all variables analyzed (percentage of insects attracted, weight of insects attracted, number of infested seeds and percentage of seed losses), as well as the percentage of emerged F2 insects, physical and bromatological analysis of corn seeds. Firstly, the genotypes were analyzed under the condition of infestation by Sitophilus zeamais, where the percentage of insects attracted to the arenas could be observed. Table 2, where the Roxo genotype showed the lowest host preference with 4.1%, followed by the AG1051 genotype which obtained 8.3%. However, the genotypes Branco (25.8%) and Palha roxa (25%) were the most attractive to insects, followed by the Glyfos RR and Vermelho genotypes with 19.1% respectively.
Lower insect preference observed in the Roxo and AG1051 genotypes may be related to the resistance mechanism called non-preference for antixenosis, which is manifested by the resistance mechanism resulting from the adverse effect of the plant on the behavior of the adult insect in herbivory and oviposition. Recent studies evaluated the susceptibility of native corn populations and weevil preference and found that genotypes presented significant differences (p ≤ 0.05) in relation to endosperm color, demonstrating that corn populations with white endosperm presented a greater number of damaged seeds, and Roxo color genotypes were less preferred with a difference of 14.6% in relation to Branco (Burgos-Díaz et al., 2020). Therefore, Guzzo et al. (2002) report that corn genotypes with specific genetic characteristics linked to grain hardness or some antinutritional factor will have less preference for herbivory or oviposition. Caused by physical barriers, constitutive chemical defenses, and indirect inducible defenses, including volatiles (Hogenhout & Bos, 2011). Regarding the weight of insects that fed on the seeds of the corn genotypes, after 96 hours, the lowest weight of insects from the Roxo genotype was observed, whose insect weight was 0.0101g, followed by the AG1051 genotype, which obtained 0.0188g, differing significantly from the other genotypes. This fact is due to the nature of the grain classified as hard or Flint, combined with the lowest content of crude protein, starch and higher hardness index. It is important to note that the Branco and Palha roxa genotypes obtained the highest weight of infested insects, 0.0329 g and 0.0311 g respectively, which may translate into greater consumption due to the high content of crude protein, starch and lower hardness of the seed, which allows the insect greater herbivory.
Lower insect weight may indicate greater difficulty for Sitophilus zeamais in boring the endosperm of the genotype Roxo and AG1051 due to the biochemical characteristics of the materials, which influenced resistance/tolerance.
Usseglio et al. (2018) found that some structures of the corn seed influence the behavior and interaction of the insect with the grain. In their research, they detected the presence of chemical components in the epicuticle that influenced recognition and attraction as a source of food and reproduction. Greater insect weight suggests greater herbivory and food availability to insects, which results in greater metabolic activity for the development or reproduction of progeny.
Also, significant differences were found for the variable number of damaged seeds and percentage of losses due to infestation of Sitophilus zeamais among the corn genotypes. There is a direct relationship between the number of seeds damaged by insects and the percentage of losses due to infestation, so that the genotypes with the lowest number of damaged seeds, such as Roxo corn and AG1051, obtained the lowest percentage of seed losses due to infestation, differing statistically from the other genotypes evaluated. In the end, the highest number of seeds damaged by Sitophilus zeamais were found in the Branco and Palha roxa genotypes and, therefore, they presented a higher percentage of seed losses due to infestation between 7.6% and 8.1% respectively, not differing statistically from each other (Table 2).
This finding leads us to believe that intrinsic factors of genetic materials such as physical hardness and chemical defenses that give a glassy consistency to corn genotypes are a resistance factor to Sitophilus zeamais capable of exerting repellency. In fact, Panizzi et al. (2009) stated that the chemical nature of grains, absence of vital nutrients, presence of volatile compounds, digestive enzymes or repellent compounds may be related to grain resistance to insect pests. In turn, Toscano et al. (1999) attributed the resistance of a cultivar to several physical, chemical and morphological characteristics that can act in a grouped or isolated way capable of attracting or repelling insects. According to Boiça Junior et al. (1997) they report that the grains present some resistance factor for non-preference or even the release of some attractive substances for preference for oviposition and feeding such as odor and flavor. Corn populations with white endosperm have a higher number of damaged seeds, and purple genotypes are less preferred, with a difference of 14.6% compared to white (Burgos-Díaz et al. 2020).
Table 2
Average values of the variable insects attracted, insect weight, number of infested seeds and seed losses of corn subjected to the Sitophilus zeamais food preference test
| Insects attracted and seed losses (g) of maize genotypes |
Genotypes | Int. Attracted (%) | PG Attracted (g) | S. Brocades | PDS (%) |
Branco | 25.8 a | 0.0329 a | 4.1 a | 7.6 a |
Glyfos RR | 19.1 b | 0.0249 b | 2.6 b | 4.6 b |
AG1051 | 8.3 c | 0.0188 c | 1.3 c | 2.2 c |
Roxo | 4.1 d | 0.0101 d | 0.8 c | 1.6 c |
Vermelho | 19.1 b | 0.0235 b | 2.8 b | 4.3 b |
Palha roxa | 25 a | 0.0311 a | 3.6 a | 8.1 a |
CV (%) | 17.6 | 19.1 | 22.9 | 26.9 |
Int. Attracted (%): Percentage of insects attracted; PG Attracted: Weight of weevils attracted (g); S. Bored: Number of bored seeds; PDS (%): Percentage of seed losses.
Means followed by the same lowercase letters in the column do not differ from each other at the 5% probability level by the Scott-Knott test.
Figure 3 shows the percentage of emerged insects (F2 progeny) in each corn genotype evaluated. A lower percentage of insects emerged was observed in the Roxo and AG1051 genotypes (5% and 6%), differing statistically from the other genotypes evaluated. A higher percentage of emerged insects were observed in the Glyfos RR (27%), Branco (22%), Palha roxa (21%) and Vermelho (19%) genotypes. A lower percentage of emerged insects in the Roxo and AG1051 genotypes may indicate resistance of the genotypes to grain insects. Both genotypes may have an antibiosis mechanism, especially due to the production of compounds that impair the development of insect larvae. This may indicate that insects do not prefer the Roxo and AG1051 genotypes regarding feeding and oviposition based on factors such as nutritional composition and seed texture. This finding had already been verified by Arnason et al. (1993) according to which the preference for a corn cultivar may be associated with factors such as pericarp resistance, the physical and/or chemical nature of the grain skin, presence of allelochemicals, phenolic substances among others interfering in the metabolism of insects.
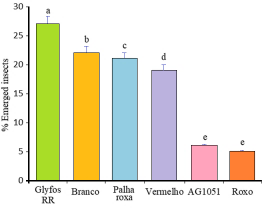
Figure 3. Percentage of emerged insects among maize genotypes. Means followed by the same lowercase letters in the column do not differ from each other at the 5% probability level by the Scott-Knott test.
The results of this research corroborate what was observed by Maggionie et al. (2016) according to which the growth of weevil populations and the damage caused are different between varieties due to the tolerance characteristics of the varieties. In this research, greater seed hardness and lower starch content were observed in the Roxo genotype, followed by AG1051, which was the first classified as having the flint or hard grain type. The resistance of creole corn to pest attacks is a characteristic observed by small farmers. Creole corn varieties may be less attacked by S. zeamais, when compared to commercial cultivars (Fernandes, 2022). Similarly, Rodríguez et al. (2022) analyzed the presence of tannins and alkaloids in stored grains and demonstrated that these compounds can be used as a form of natural resistance. For example, studies carried out by Zhang et al. (2021) observed the preference of insects for rice grains based on chemical composition, evidencing that the presence of certain specific phenolic and volatile compounds can reduce insect attraction.
Analysis of the centesimal and physical composition of the corn genotypes showed significant differences for all the characteristics evaluated. The crude protein contents (%) were higher in the Branco genotype, obtaining 10.7%, followed by the Palha roxa genotype (9.6%). These two genotypes were the ones that presented the greatest preference of the insects, more damaged seeds and the highest percentage of seed losses, this fact demonstrates a direct relationship between the increase in protein and insect attractiveness. For the ethereal extract (%) three genotypes stood out, Branco with 5.5%, Glyfos RR obtained 5.2% and Vermelho with 5.2%, with a lower percentage of ethereal extract being found in the genotypes Roxo, AG1051 and Palha roxa.
Regarding crude fiber (%), the Branco genotype stood out, obtaining 3.1%, differing from the other genotypes. The same genotype (Branco) obtained a higher starch content, obtaining 58.3%. This content can characterize the material as having a more starchy endosperm and associated with the protein content, it is more preferred and consequently more bored by insects.
The Roxo genotype had a lower starch content (52.7%), which may have contributed to the mechanism of non-feeding preference. The same genotype obtained 78.1 HK of seed hardness, which demonstrates that physical hardness is related to the capacity of the endosperm to resist the weevil. This genetic trait can be used as a source of resistance to improve varieties against the attack of Sitophilus zeamais or mass production of this variety in communities where control of stored grains constitutes challenges in the post-harvest program. Corn genotypes with higher crude protein content, starch content and lower physical hardness of the seeds are more susceptible to infestation by Sitophilus zeamais and, therefore, present a higher percentage of seed losses. On the one hand, they constitute a protein source in the human diet, but on the other hand, they require rigorous methods for seed and grain conservation due to their preference and susceptibility to attack by Sitophilus zeamais (Table 3).
Studies conducted by Antunes et al. (2011) found that the protein content in corn varieties was 9.11%. However, Pacheco (2009) found that the proteins in corn have a composition of 4% albumin, 2% globulin, 55% prolamin and 39% glutelin. These protein values in corn seeds are similar to those found in the present study, with the White genotype having a greater emphasis on protein content of 10.7%. Antunes et al. (2011) found that corn varieties are more affected by fat content and did not find significant differences in protein content. This is because, according to Puzzi (1986), insects feed on endosperm in the larval phase and then, in the adult phase, on the germ, which causes considerable weight loss and loss of germination power of the seeds. In the present study, it was found that genotypes with higher protein, fat, starch content and lower hardness index were more preferred by insects.
Figure 4 shows the Person correlation graph between the variables analyzed. Through Person correlation, it was possible to distinguish three large inversely correlated groups. The first group represents a significant, strong and positive correlation between attracted insects and weight of attracted weevils in the genotypes (0.95); bored seeds (0.94); percentage of seed losses (0.92); percentage of crude protein (0.50); attracted insects and percentage of starch (0.65).
This analysis explains that the nutritional composition of the analyzed genotypes favors the attraction or repellency of insects, which results in a higher percentage of seed losses associated with protein and starch contents. Thus, more starchy and protein-rich genotypes are preferred for insect feeding and oviposition, given the protein values of the endosperm and germ.
On the other hand, the second group includes the significant, weak and positive correlation between attracted insects and seed hardness index (-88), this relationship demonstrates that the seed hardness of the genotypes exerts a great influence on food preference and oviposition.
Genotypes with greater endosperm hardness and lower starch and protein content are more resistant to Sitophilus zeamais attack, resulting in lower weevil weight (-077), lower percentage of bored seeds (-0.85) and consecutively lower seed losses (-088). The third group represents (non-significant and weak correlation) between attracted insects and ether extract (0.15) and between attracted insects and crude fiber (0.29). Although crude fiber did not present a correlation with the percentage of attracted insects, it had a significant, strong and positive correlation with crude protein (0.72). The results of this research corroborate what was observed by Maggionie et al. (2016) according to which the growth of weevil populations and the damage caused are different between varieties due to the tolerance characteristics of the varieties.
Table 3
Average values of the variable crude protein, ether extract, crude fiber, starch and hardness of corn seed submitted to the Sitophilus zeamais food preference test
| Bromatological and physical characteristics of maize genotypes |
Genotypes | Gross P (%) | E. Ethereal (%) | Gross F (%) | Starch (%) | Hardness (HK) |
Branco | 10.7 a | 5.5 a | 3.1 a | 58.3 a | 62.3 c |
Glyfos RR | 8.7 ab | 5.2 a | 2.3 c | 55.2 b | 62.1 c |
AG1051 | 9.3 c | 4.9 b | 2.3 c | 56.2 b | 76.1 b |
Roxo | 9.1d | 4.8 b | 2.5 b | 52.7 c | 78.1 a |
Vermelho | 9.1 d | 5.2 a | 2.6 b | 55.9 b | 62.1 c |
Palha roxa | 9.6 b | 4.5 c | 2.3 c | 56.4 b | 61.3 c |
CV (%) | 0.8 | 4.4 | 5.8 | 1.6 | 4.4 |
Crude P. (%): Percentage of crude protein; E. Ethereal (%): Percentage of ethereal extract; F. Crude (%): Percentage of crude fiber; Starch (%): Percentage of starch; Hardness (HK) seed. Means followed by the same lowercase letters in the column do not differ from each other at the 5% probability level by the Scott-Knott test.
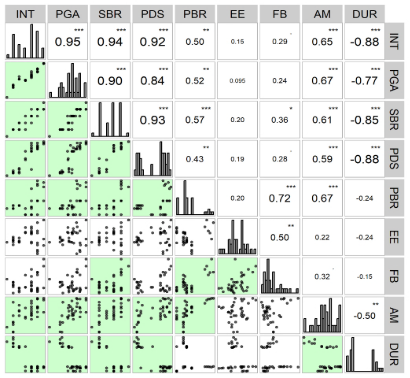
Figure 4. Pearson correlation graph between attracted insects, insect weight, number of infested seeds and centesimal analysis of maize genotypes. Not significant p > 0.05; * Significant p < 0.05%; ** Significant p < 0.01%; *** Significant p < 0.1%. INT: Insects Attracted; PGA: Weight of Weevils (g); SBR: Bored Seeds; PDS: Percentage of Seed Loss; PBR: Percentage of Crude Protein; EE: Ether Extract; FB: Crude Fiber; AM: Percentage of Starch; DUR: Physical Hardness of Seed.
Resistance of the pericarp, the physical and/or chemical nature of the grain film, presence of allelochemicals, phenolic substances among other plant resistance factors directly interfere in the metabolism of insects (Arnason, 1993).
4. Conclusions
The maize genotypes present different mechanisms of non-feeding preference and oviposition by S. zeamais, with emphasis on Roxo maize, which presented antixenosis resulting from the effect of the nutritional properties and physical hardness of the seed, which resulted in lower attractiveness and seed losses due to attack by.
The result suggests the selection of genotypes with a non-preference or antixenosis mechanism, as it has been shown to be a promising strategy for improving seed conservation systems in organic and family farming.
Based on the results obtained in this research, further studies are needed to analyze the biochemistry of grain insects that aim to understand the antagonistic effects of maize genotypes on the preference of S. zeamais as a source of resistance to infestation.
Acknowledgements
The authors would like to thank the Federal University of Espirito Santo (UFES) for providing quality teaching and research, the laboratory technicians, experimental area staff and other collaborators; To the technicians and researchers at the seed analysis and centesimal composition laboratory at Embrapa-Milho e Sorgo who helped with the bromatological determination. Thanks are extended to the Coordination for the Improvement of Higher Education Personnel - Brazil (CAPES) and the Espírito Santo Research and Innovation Support Foundation (FAPES) - for granting the master's and doctoral scholarship.
Author Contribution
R. A. Marcos: Experimental planning, implementation and execution of the experiment, data collection and analysis, writing and formatting. F. G. Holtz: Experiment implementation, data collection and language translation. M. M. O. Ramos: Implementation of the experiment and data collection. J. A. da Silva: Data analysis, writing and review. M. L. F. Simeone: NIRs calibration, grain proximate analysis, data interpretation and writing. H. B. Zago: Guidance, methodological definition, work review and validation. F. L. De Oliveira: Guidance, data review, validation and journal selection. L. P. Dalvi: Methodological guidance, project administration, supervision, validation, visualization, writing – original draft, writing, review and validation.
Conflict of interest statement
Authors have no conflict of interest to declare.
ORCID
R. A. Marcos https://orcid.org/0000-0001-6680-9679
F. G. Holtz https://orcid.org/0000-0001-9669-0189
M. M. O. Ramos https://orcid.org/0009-0007-5179-4188
J. A. da Silva https://orcid.org/0000-0001-6921-6043
M. L. F. Simeone https://orcid.org/0000-0002-2003-0341
H. B. Zago https://orcid.org/0000-0003-1975-3590
F. L. De Oliveira https://orcid.org/0000-0002-1711-6988
L. P. Dalvi https://orcid.org/0000-0002-2995-8007
References
Antunes, L. E., Viebrantz, P. C, Gottardi, R., & Dionello, R. G. (2011). Características físico-químicas de grãos de milho atacados por Sitophilus zeamais durante o armazenamento. Revista Brasileira de Engenharia Agrícola e Ambiental, 15, 615-620. https://doi.org/10.1590/S1415-43662011000600012
Alves, L. S., & Poltronieri, A. S. (2024). Resistência de três genótipos de milho ao ataque de Sitophilus zeamais Motschulsky, 1885 (Coleoptera: Curculionidae) em laboratório. Research, Society and Development, 13(5), e129135454864. https://doi.org/10.33448/rsd-v13i5.45864
Arnason, J. T., Baum, B., Gale, J., Lambert, J. D. H., Bergvinson, D., Philogene, B. J. R., & Jewell, D. C. (1993). Variation in resistance of Mexican landraces of maize to maize weevil Sitophilus zeamais, in relation to taxonomic and biochemical parameters. Euphytica, 74. https://doi.org/10.1007/BF00040405
Boiça JR, A. L., Lara, F. M., & Guidi, F. P (1997). Resistance of corn genotypes to attack by Sitophilus zeamais Mots. (Coleoptera: Curculionidae). Annals of the Entomological Society of Brazil, 26, 481-485. https://doi.org/10.1590/S0301-80591997000300010
Burgos-Díaz, J. A., Rangel-Fajardo, M. A., Tucuch-Haas, J. I., Benítez-Riquelme, I., & García-Zavala, J. J. (2020). Susceptibility of native maize populations and preference of the weevil in Yucatán, México. Revista mexicana de ciencias agrícolas, 11(7), 1469-1479. https://doi.org/10.29312/remexca.v11i7.2081
Brasil. (2009). Rules for seed analysis. Ministry of Agriculture, Livestock and Food Supply. Secretariat of Agricultural Defense. Brasília, DF: Mapa, 395p. https://www.gov.br/agricultura/pt-br/assuntos/insumos-agropecuarios/arquivos-publicacoes-insumos/2946_regras_analise__sementes.pdf
Conceição, E. D. R. S., de Souza David, A. M. S., Paraizo, E. A., Soares, L. M., Figueiredo, J. C., Alvarenga, C. D., ... & da Silva, J. P. M. (2024). Quality of corn seeds infested with Sitophilus zeamais after application of plant extracts and diatomaceous earth. Caderno Pedagógico, 21(13), e11587-e11587. https://doi.org/10.54033/cadpedv21n13-102
Compton, J. A., Floyd, S., Ofosu, A., & Agbo, B. (1998). The modified count and weigh method: An improved procedure for assessing weight loss in stored maize cobs. Journal of Stored Products Research, 34(4), 277-285. https://doi.org/10.1016/S0022-474X(98)00009-5
Cruz, I. Cruz, J. C., Karam, D., Monteiro, M. A. R., & Magalhaes, P. C. (2008). A cultura do milho. Sete Lagoas: Embrapa Milho e Sorgo, 2008. cap. 12, p. 303-362.
Fazolin, M., da Costa, C. R., Damaceno, J. E., de Albuquerque, E. S., Cavalcante, A. S. da S., Estrela, J. L. V. (2010). Fumigation of maize for weevil control using Tanaecium nocturnum (Bignoniaceae). Pesq. agropec. bras., 45(1). https://doi.org/10.1590/S0100-204X2010000100001
Frazão, C. A. V., Silva, P. R. R., Almeida; W. A. de., Pontual, E. V., Cruz, G. dos S., Napoleão, T. H., & França, S. M. de. (2018). Resistance of maize cultivars to Sitophilus zeamais (Coleoptera: Curculionidae). Arquivos do Instituto Biológico, 85, 1-8. https://doi.org/10.1590/1808-1657000552017
Fernandes, G. B. (2022). Dynamic conservation of maize landraces by family farmers in Minas Gerais, Brazil. Agrociencia Uruguay, 26(NSPE3), e959. https://doi.org/10.31285/agro.26.959
Gallo, D., Nakano, O. N., Silveira Neto, S., Carvalho, R. P. L. C., Batista, G. C. D., Berti Filho, E., Parra, J. R. P., & Zuchi, R. A. (2002). Entomologia agrícola. Piracicaba; FEALQ. p. 839-840.
García-Lara, S., Espinosa Carrillo, C., & Bergvinson, D.J. (2007). Manual de Plagas en Granos Almacenado y Tecnologías Alternas para su Manejo y Control; CIMMYT: El Batán, Mexico.
Guzzo, E. C., Alves, L. F. A., Zanin, A., & Vendramin, J. D. (2002). Identification of corn materials resistant to attack by the weevil Sitophilus zeamais (Mots., 1855) (Coleoptera: Curculionidae). Archives of the Biological Institute, 69(2), 69-73. https://doi.org/10.1590/1808-1657v69n2p0692002
Hogenhout, S. A., & Bos, J. I. (2011). Effector proteins that modulate plant–insect interactions. Current opinion in plant biology, 14(4), 422-428.https://doi.org/10.1016/j.pbi.2011.05.003
Kim, S. K., & Kossou, D. K. (2003). Genetic responses of resistant maize germplasm to the maize weevil Sitophilus zeamais Motschulsky in West Africa. J. Prod. Stored Res., 39, 489–505. https://doi.org/10.1016/S0022-474X(02)00056-5
Maggioni, K., Silva, L. B., Xavier, Z. F., de Bortoli Munhae, C., Dourado, L. R. B., & Pavan, B. E. (2016). Performance of populations of Sitophilus zeamais Motschulsky (Coleoptera: Curculionidae) on different varieties of maize. African Journal of Agricultural Research, 11(10), 873-881. https://doi.org/10.5897/AJAR2015.10505
Muzemu, S., Chitamba, J., & Goto, S. (2013). Screening of stored maize (Zea mays L.) varieties grain for tolerance against maize weevil, Sitophilus zeamais (Motsch.). International Journal of Plant Research, 3(3), 17-22. https://doi.org/10.5923/j.plant.20130303.01
Nwosu, L. C. (2016). Chemical bases for maize grain resistance to infestation and damage by the maize weevil, Sitophilus zeamais Motschulsky. Journal of Stored Products Research, 69, 41-50. https://doi.org/10.1016/j.jspr.2016.06.001
Pacheco, F. P. F. (2009). Storage proteins and preference of Sitophilus sp. for local and improved rice (Oryza sativa L.) varieties grown under different soil management conditions. Dissertação Mestrado em Agroecologia. Universidade Estadual do Maranhão.
Panizzi, A. R., & Parra, J. R. P. (2009). Bioecology and nutrition of insects: basis for integrated pest management. Brasília/DF: Embrapa information technology. 164 p.
Puzzi, D. (1986). Grain supply and storage. São Paulo: Campineiro Institute of Agricultural Education.
R Core Team (2021). R: A language and environment for statistical computing. R Foundation for Statistical Computing. https://www.R-project.org/. https://doi.org/10.59350/t79xt-tf203
Rodríguez, A., Beato, M., Usseglio, V. L, Camina, J., Zygadlo, J. A., Dambolena, J. S, & Zunino, M. P (2022). Phenolic compounds as controllers of Sitophilus zeamais: A look at the structure-activity relationship. Journal of Stored Products Research, 99, 102038. https://doi.org/10.1016/j.jspr.2022.102038
Rossetto, C. J. (1972). Resistance of corn to ear pests, Helicoverpa zea (Boddie), Sitophilus zeamais Motschulsky and Sitotroga cerealela (Oliver). 111f. Thesis (Doctorate in Agronomy) - Luiz de Queiroz College of Agriculture, University of São Paulo, Piracicaba.
Simeone, M. L. F., Pimentel, M. A. G., Queiroz, V. A. V., Santos, F., Brito, A., Aquino, L. F. M., ... & Trindade, R. D. S. (2024). Portable near-infrared (NIR) spectroscopy and multivariate calibration for reliable quality control of maize and sorghum grain chemical composition. Journal of Food Composition and Analysis, 134, 106502. https://doi.org/10.1016/j.jfca.2024.106502
Toscano, L. C., Boiça Jr, A. L., Lara, F. M., & Waquil, J. M. (1999). Resistance and mechanisms involved in corn genotypes in relation to attack by the weevil, Sitophilus zeamais Mots. (Coleoptera: Curculionidae). Annals of the Entomological Society of Brazil, 28, 141-146. https://doi.org. 10.1590/S0301-80591999000100015
Usseglio, V. L., Dambolena, J. S., Merlo, C., Peschiutta, M. L., & Zunino, M. P. (2018). Insect-corn kernel interaction: chemical signaling of the grain and host recognition by Sitophilus zeamais. Journal of stored products research, 79, 66-72. https://doi.org/10.1016/j.jspr.2018.08.002
Zhang, Y., Teng, B., Wang, D., & Jiang, J. (2021). Discovery of a specific volatile substance from rice grain and its application in controlling stored-grain pests. Food Chemistry, 339, 128014. https://doi.org/10.1016/j.foodchem.2020.128014
. Where PSnd: weight of undamaged seeds; Pfa: final weight of the sample.
.