RESEARCH ARTICLE
Phytophthora species causing root rot in avocado seedlings at Colombian nurseries: Morphological, molecular, and pathogenic analysis
Lizeth Paola Palacios-Joya1 ; Kevin Alejandro Rodríguez-Arévalo1 ; Mauricio Fernando Martínez1 ;
Nubia Murcia-Riaño1 ; Diana Milena Rodríguez-Mora1 *
1 Corporación Colombiana de Investigación Agropecuaria-AGROSAVIA, C.I. Palmira. Palmira (Valle del Cauca) 763537, Colombia.
* Corresponding author: dmrodriguez@agrosavia.co (D. M. Rodríguez-Mora).
Received: 14 June 2024. Accepted: 1 January 2025. Published: 28 January 2025.
Abstract
Expansion of avocado production areas in Colombia has led to an increased demand for plant propagation material. However, this expansion has exacerbated phytosanitary challenges, particularly root rot disease mainly associated with Phytophthora spp. Therefore, this study aimed to identify Phytophthora species associated with root rot in avocado seedlings within nurseries. Avocado plants exhibiting wilting symptoms were collected from nurseries in the departments of Quindío, Risaralda, and Valle del Cauca (Colombia). Segments of diseased roots were selected, cut, and surface-disinfected, before being planted on Potato Dextrose Agar (PDA) supplemented with antibiotics and fungicides. Microorganism identification was conducted using taxonomic keys and confirmed by molecular techniques employing the identification based on phylogenetic hypothesis, using ITS1-5.8s-ITS2 region encoding for rRNA. Isolates obtained from necrotic avocado roots were identified as P. cinnamomi and P. heveae. The pathogenicity of the isolates was confirmed in avocado seedlings through inoculation, resulting in symptom reproduction. Consequently, this study identified P. cinnamomi and P. heveae as causal agents of root rot in avocado during the nursery stage.
Keywords: PCR; Phytophthora cinnamomi; P. heveae; Persea americana; wilting.
DOI: https://doi.org/10.17268/sci.agropecu.2025.010
Cite this article:
Palacios-Joya, L. P., Rodríguez-Arévalo, K. A., Martínez, M. F., Murcia-Riaño, N., & Rodríguez-Mora, D. M. (2025). Phytophthora species causing root rot in avocado seedlings at Colombian nurseries: Morphological, molecular, and pathogenic analysis. Scientia Agropecuaria, 16(1), 113-121.
1. Introduction
The primary limitation for avocado (Persea americana Mill.) production worldwide are root diseases. Among the pathogens associated with this issue is Phytophthora cinnamomi Rands (Oomycota: Peronosporales: Peronosporaceae) (Álvarez et al., 2023). This pathogen has been globally described as causal agent of root rot, a symptom that triggers chlorosis in leaves and plant wilting. The disease progresses with defoliation and dieback of the tree, leading to a reduction in production (Kavroulakis et al., 2024).
Phytophthora cinnamomi has severely affected avocado orchards in Florida and California (USA), causing economic losses of nearly USD $40 million (Belisle et al., 2019). In Greece, the first report of this disease was made in 2024, where it showed a high mortality rate (Kavroulakis et al., 2024). While in Mexico, it caused the death of 100,000 'Hass' avocado trees in Uruapan, Michoacán, with producers facing economic losses exceeding 32 million pesos (Agapito et al., 2022). Phytophthora cinnamomi has also been identified as the causative agent of avocado root rot in various regions and countries, including North America, Latin American, Australia, New Zealand, South Africa, Spain, Morocco, and Israel (Agapito et al., 2022).
In Colombia, avocado crop has experienced a rapid surge in planting areas, escalating from 36,461 ha to 110,183 ha between 2015 and 2022 (FAOSTAT, 2024). The expansion of cultivation areas in this country has been propelled by the goal of positioning itself as a leading exporter of Hass avocados in international markets (Álvarez et al., 2023).
The Root rot caused by P. cinnamomi has been reported in several avocado-producing departments at Colombia. This pathogen causes losses in commercial crops ranging from 45% to 90% (Ramírez-Gil, 2018). During the nursery stage an incidence of 28 % was recorded, while mortality, measured as the number of plants that died due to the disease, reached 60% (Ramírez-Gil et al., 2017). Conditions in nurseries, such as reduced spacing, constant irrigation, high humidity, and excessive use of fertilizers, create an environment conductive to the onset of root rot, leading to the propagation of plant material with low physiological and phytosanitary quality (Ramírez-Gil, 2018).
The expansion of avocado cultivation areas conducts a series of technological challenges related to phytosanitary management, where the health of planting material is crucial to ensure the sustainability of the crop and responding to the demands of producers (Ramírez-Gil, 2018). Therefore, the aim of this study was to identify, through morphological, molecular, and pathogenic analyses, Phytophthora species causing root rot in avocado plants in nurseries. This represents a crucial initial step in establishing integrated management alternatives that are economically viable, socially responsible, and environmentally sustainable.
2. Methodology
2.1 Study Area and Sample Collection
Sampling was carried out in 30 avocado-producing nurseries, with 16 located in the Valle del Cauca department, nine in Quindío, and six in Risaralda (Colombia). A total of 138 plants were collected under a collection permit issued by the National Environmental Permits Authority of Colombia (Resolution No. 1466 - December 3, 2014, https://doi.org/10.15472/gcavk3). The avocado plants collected in nurseries showed symptoms such as aerial wilting, chlorosis, and partial root rot, accompanied by a scant volume of roots (Figure 1). The collected samples were processed at Agricultural Microbiology Laboratory of the Corporación Colombiana de Investigación Agropecuaria - AGROSAVIA, Centro de Investigación Palmira.
2.2 Isolation of microorganisms
Avocado roots were first washed with tap water to remove excess soil. Subsequently, 0.5 cm pieces were cut, incorporating both healthy and necrotic tissue. The tissue underwent superficial disinfection with 0.1% sodium hypochlorite for 45 seconds, followed by 70% alcohol for one minute, and then rinsed three times with sterile distilled water. The tissue was placed on sterile paper, air-dried, and then planted on Potato Dextrose Agar (PDA) supplemented with antibiotics and fungicides, following the method described by Tsao & Guy (1983). Isolates were purified by hyphal tip method (Abad et al., 2023).
2.3 Morphological characterization
Morphological identification of the isolates was carried out using the taxonomic keys in the IDphy tool (https://idtools.org/tools/1056/index.cfm) (Abad et al., 2023). The isolates were morpholo-gically characterized on PDA medium, where macroscopic features such as the type of mycelial growth, colony color, and shape were assessed.
The microscopic characterization of the isolates was performed using a Nikon ECLIPSE Ci-L microscope, image capture was carried out with the Nikon DS-Ri2 camera, and the NIS-Elements D software was used. Microscopic characteristics including hyphae, chlamydospore shape, and sporangial features such as shape, dimensions (length and width), number of papillae, and type of papillation were also evaluated. For homothallic isolates, the formation of oogonia, along with their size, shape, and surface characteristics were assessed.
2.4 DNA Extraction and PCR
For molecular identification, total genomic DNA was extracted from mycelium grown on PDA medium using the protocol of Raeder & Broda (1985), whit some modifications as described by Ángel-García et al. (2023). The region ITS1-5.8s-ITS2, encoding for rRNA, was amplified by Polymerase Chain Reaction (PCR) using primers ITS1 (5'-TCCGTAGGTGAACCTGCGG-3') and ITS4 (5'-TCCTCCGCTTATTGATATGC-3') (White et al., 1990). Each reaction was performed with 10 ng of genomic DNA, 1X Taq Buffer with KCl, 1.5 mM MgCl2, 0.2 mM dNTPs, 0.2 µM of each primer, 1U Taq DNA polymerase (recombinant) (Thermo Scientific), for a final volume of 25 µl. The PCR was carried out in an Agilent SureCycler 8800 thermocycler with the following thermal profile: an initial cycle at 94 °C for 5 min, followed by 35 cycles at 94 °C for 45 seconds, 55 °C for 30 seconds, 72 °C for 1 min, and a final step at 72 °C for 10 min.
2.5 Sequencing and phylogenetic analysis
PCR products were sequenced in both forward and reverse directions using the Sanger method. Sequence cleaning and assembly were carried out using Geneious 2020.2.1 software. Alignment was conducted with Muscle v3.8.1551 software. Representative ex-type sequences of Phytophthora species reported worldwide, belonging to different clades, were included. Sequences were retrieved from GenBank database as reported by Abad et al. (2023).

Figure 1. Symptoms observed in sampled West Indian avocado plants in nurseries; a) Generalized wilting of the plant; b) Aerial wilting, chlorosis, and dry leaves; c) and d) Partial necrosis of roots. Photographs: Research Group of AGROSAVIA, Centro de Investigación Palmira.
The optimal base substitution model for the data was determined using the ModelTest-NG software version 0.1.6 employing the Bayesian information criterion (BIC). Construction of the Phylogenetic tree was executed by Maximum Likelihood method using PhyML software v3.3.20190909. The internal topology support of the dendrograms was assessed through bootstrap analysis with 1,000 replications. Visualization of the Phylogenetic tree was performed in R software using ggtree library.
2.6 Pathogenicity tests
Pathogenicity tests were conducted on three-month-old Hass avocado plants derived from seeds and maintained under plastic cover and shade with a 50% reduction in light. The isolates were cultured on PDA medium and incubated for 15 days at 28 °C. Inoculation was carried out using the stem-wounding method, following the procedure outlined by Dixon et al. (1984): cuts were made 8 cm from the base of the stem, where a 5 mm Ø PDA disk with mycelial growth of the isolate was inserted and covered with Parafilm (Figure 2). For the control treatment, PDA disks without mycelial growth were used.

The experiment was arranged in a completely randomized design (CRD) with one plant as experimental units and six replications assigned to each treatment (isolate).
Figure 2. Diagram of the pathogenicity test.
Evaluations were conducted at 3, 6, 9, 12, 15, 18, 21, 24 and 27 days a post-inoculation (dpi), deter-mining the degree of severity based on the visual symptom scale designed for P. cinnamomi in Hass avocado by Ramírez & Morales (2020), the size of the necrotic lesions was also recorded and the Area Under the Disease Progress Curve (AUDPC) was determined. To confirm Koch's postulates, necrotic stem tissue was cultured on PDA medium, and microscopic observations were conducted for isolate identification. An analysis of variance (ANOVA) and mean comparison using the Duncan test (p < 0.05) were performed with the average ABCPE using R software, Version: 4.3.2.
The trial was conducted under aphid-proof mesh house conditions, with periodic watering every three days. The relative humidity ranged between 46.9% and 59.7%, and temperatures fluctuated between 24.6 °C and 26.3 °C. Environmental conditions were monitored using a WatchDog meteorological station, programmed to record variables every 30 minutes for 27 days.
3. Results and discussion
Isolation of microorganisms
Out of the 138 processed avocado plants, 11 isolates of Phytophthora sp. were obtained, with 10 originating from nurseries in Valle del Cauca and one from a nursery in Quindío (Table 1). This study found a low frequency of Phytophthora sp. isolates, a finding consistent with reports by Saltarén et al. (1998). However, these results differ from those obtained by Ramírez-Gil et al. (2017), who identified P. cinnamomi as the main causal agent of the wilting complex in avocado plants in nurseries in the department of Antioquia, with a frequency of 45 %, followed by abiotic problems such as hypoxia and anoxia. The scarce presence of Phytophthora sp. in the analyzed nurseries could be related to the employed management practices, including the use of sterile substrates and the application of active ingredients such as Dazomet for substrate disinfection, aimed at controlling fungi and oomycetes (Villarino et al., 2021). Additionally, the use of biological products based on Trichoderma spp. may impact colony-forming units of Phytophthora sp. (Leal et al., 2014). Likewise, it’s conceivable that the sampled avocado plants in the nurseries may be affected by other causal agents associated to root rot (Parkinson et al., 2017).
Morphological characterization
Out of the 11 isolates of Phytophthora sp. obtained from necrotic roots of avocado plants produced in the nurseries, six isolates were selected for morphological and molecular identification as well as pathogenic characterization. Morphological characterization revealed that three isolates were identified as P. cinnamomi (Per2PH, Profru1PH, Ses4PH) and three isolates as P. heveae A.W. Thomps. (Profru2PH, Profru4PH, Rena1PH).
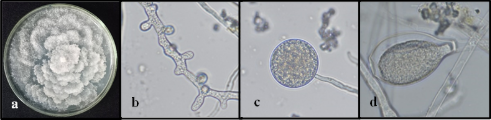
Distinctive patterns were observed between the two Phytophthora species through morphological analysis. Phytophthora cinnamomi showed colonies with trailing mycelium, petaloid pattern, the presence of hyphal swellings and chlamydospores, unbranched sporangiophores, and non-papillate sporangia, with ellipsoidal or lemon-shaped forms (Figure 3), with lengths between 48–60 µm (Table 1). Phytophthora heveae displayed colonies with aerial mycelium, absence of hyphal swellings or chlamydospores, its sporangiophores have a simple sympodial branching pattern, with papillate sporangia (1 to 2 papillae) of globular or ovoid shape (Figure 4) and with lengths between 34–39 µm (Table 1), making them smaller compared to the sporangia of P. cinnamomi. These observed characteristics align with previous reports by Abad et al. (2023).
Analysis phylogenetic
Phytophthora spp. molecular identification was carried out by amplifying the ITS region of ribosomal DNA using the primers ITS1 and ITS4. The six consensus sequences generated in this study have been deposited in GenBank for reference and public access.
Figure 3. Macroscopic and microscopic appearance of P. cinnamomi on PDA medium. a) Trailing white mycelium with a petaloid pattern; b) Hyphal swellings (40x); c) Globose chlamydospores with thin walls (40x); d) Non-papillate ellipsoidal sporangium (40x). Photographs: Research Group of AGROSAVIA, Centro de Investigación Palmira.
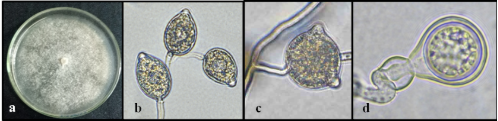
Figure 4. Macroscopic and microscopic appearance of P. heveae on PDA medium. a) Trailing white mycelium without a characteristic pattern; b) Sporangiophore with simple sympodial branching and ovoid papillate sporangia (40x); c) Globose sporangium with two papillae (40x); d) Globose oogonium with a conical base and round oospore, amphigynous antheridium (100x). Photographs: Research Group of AGROSAVIA, Centro de Investigación Palmira.
Table 1
Macro and microscopic characterization of isolates of P. cinnamomi and P. heveae
Isolated | Location | Phytophthora specie | Colony | Asexual phase | Sexual phase |
CP | TC | CC | HS | FC | SF | SP | NP | SL | SW | SH | ER | OW | AA | TO |
Per2PH | Valle del Cauca | P. cinnamomi | P | C | W | HS | CH | UN | NP | 0 | 49,9 | 33 | EL | HT | - | - | - |
Profru1PH | Valle del Cauca | P. cinnamomi | NP | C | W | HS | CH | UN | NP | 0 | 57,3 | 33,4 | EL | HT | - | - | - |
Ses4PH | Valle del Cauca | P. cinnamomi | P | C | W | HS | CH | UN | NP | 0 | 48,2 | 32,7 | EL | HT | - | - | - |
Profru2PH | Valle del Cauca | P. heveae | NP | A | W | - | - | SS | P | 2 | 37,4 | 24,8 | GO | HO | S/tb | A | 21,9 |
Profru4PH | Valle del Cauca | P. heveae | NP | A | W | - | - | SS | P | 2 | 34,2 | 24,6 | GO | HO | S/tb | A | 20,3 |
Rena1PH | Quindío | P. heveae | NP | A | W | - | - | SS | P | 1 | 38,3 | 25,1 | OI | HO | S/tb | A | 20 |
CP Colony Pattern NP = Not Present, P = Petaloid, TC Colony growth type C = Creeping, A = Aerial, CC Colony color W= White, HS Formation of hyphal swellings HS = formed,"-" = not formed., FC Formation of chlamydospores CH = formed, CH/- = sometimes formed, and "-" = not formed., SF Sporangiophore form SS = Simple sympodial, UN = unbranched, SP Sporangia papillae P = papillate, SP = semi-papillate, NP = non-papillate., NP Number of papillae 0 = No papillae, 1 = one papillae, 2 = one and two papillae, SL Sporangia length (µm), SW Sporangia width (µm), SH Sporangia shape E= ellipsoid, O = ovoid, L = limoniform, G = globose, I = irregular, RE Reproductive strategy HO = homothallic and HT = heterothallic., OW Oogonial wall S/tb = smooth wall and tapered base, "-" = was not determined, AA Antheridial attachment P = paragynous, A = amphigynous, and "-" = was not determined, OS Oospore size (diameter µm), and "-" = was not determined.
For P. cinnamomi, the accession numbers OR378296, OR378297, and OR378303 were assigned for Per2PH, Profru1PH and Ses4PH, respectively. For P. heveae, the accession numbers OR378298, OR378299 and OR378301 were assigned for Profru2PH, Profru4PH and Rena1PH, respectively.
Phylogenetic analyses of the complete ITS region dataset for three isolates of P. cinnamomi showed a clear clustering with the ex-type strain CPHST BL 12 (MG865473) and different isolates obtained from avocado plants (Figure 5). Three isolates clustered with the ex-type strain of P. heveae (MH136899), alongside isolates from Ecuador, Guatemala, and Hawaii. The constructed tree exhibited a similar topology and high cladistic consistency for Phytophthora species, where P. cinnamomi is included in clade 7a and a P. heveae belongs to clade 5 (Abad et al., 2023; Lanete et al., 2023).
Pathogenicity tests
In the pathogenicity tests conducted with isolates of P. cinnamomi and P. heveae on Hass avocado seedlings, symptoms similar to those observed in nurseries were reproduced. Symptoms of infection and pathogen colonization during the first six days post-infection (dpi) manifested as wilting and chlorosis of the seedlings (Figure 6a and c). Between nine and 12 dpi, the seedlings developed symptoms of wilting and downward death (Figure 6b and d). Plants in the control treatment showed no symptoms and continued with normal growth throughout the evaluation period (Figure 6e). These results are consistent with those reported by Marulanda (2018), who evaluated a collection of P. cinnamomi isolates in Hass avocado plants using the stem wound method, where symptoms described as wilting, and chlorosis were observed between the third and sixth dpi. P. cinnamomi and P. heveae were re-isolated from necrotic lesions, and in all cases, the identity of the oomycetes was confirmed, supporting the pathogenic nature of both Phytophthora species in avocado plants.
The analysis of variance conducted on the AUDPC of the six evaluated isolates showed highly significant differences (p < 0.001), confirming variability in the pathogenicity of the Phytophthora spp. isolates. Three groups of means with highly significant differences (p < 0.001) were formed, as depicted in Figure 7. Isolate Per2PH (P. cinnamomi) exhibited higher AUDPC and necrotic lesions area than other isolates, thereby emerging as the most virulent in this study. Profru2PH (P. heveae), exhibited an average AUDPC of 2,117 and an average necrotic lesion area of 10.1 cm2, did not differ from isolates Rena1PH (P. heveae), Ses4PH (P. cinnamomi), and Profru1PH (P. cinnamomi) means.
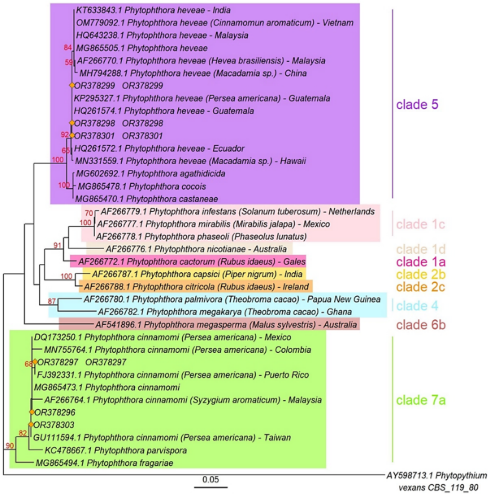
Figure 5. Phylogenetic tree of Phytophthora spp. isolates, generated with sequences of the ITS1-5.8s-ITS2 region, obtained by maximum likelihood method. The tree was constructed using the PhyML software (bootstrap=1,000), employing the Tim3 substitution model including a gamma distribution rate (+G4). Bootstrap values are located above the branches.

Figure 6. Symptoms observed in three-month-old Hass avocado plants inoculated with Phytophthora spp. a) Chlorosis and wilting caused by P. cinnamomi six dpi, and b) Wilting and death caused by P. cinnamomi, 15 dpi. c) Wilting caused by P. heveae, six dpi. d) Wilting and death caused by P. heveae nine dpi. e) Control treatment. Photographs: Research Group of AGROSAVIA, Centro de Investigación Palmira.
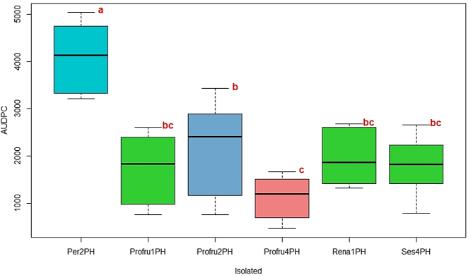
Figure 7. Boxplot of the Area Under the Disease Progress Curve (AUDPC) in Hass avocado seedlings inoculated with different isolates of Phytophthora spp.
However, it did differ from Per2PH and Profru4PH. The isolates Rena1PH (P. heveae), Ses4PH (P. cinnamomi), Profru1PH (P. heveae), and Profru4PH (P. heveae) did not show significant differences among them. They exhibited intermediate infective capacity and lower infection speed, with an average AUDPC ranging between 1,961 and 1,121 and an average necrotic lesion area ranging between 7.4 cm2 and 8.5 cm2. The control treatment (mock inoculation) did not produce necrotic lesions or any other symptoms.
Isolates Per2PH and Profru2PH of P. cinnamomi and P. heveae exhibited a faster symptom expression rate and seedling mortality in this study. At 15 dpi, 83% of the material inoculated with these two isolates died. Profru2PH showed more virulence, affecting between 30% and 50% of the inoculated material within the first six to nine dpi. Isolate Rena1PH caused the death of 33% of the material at six dpi and 50% at 15 dpi. Isolates Profru1PH and Profru4PH demonstrated lower virulence, causing only mild wilting in the plants without resulting in mortality during the evaluation period. These findings indicate variability in the virulence of the isolates, which aligns with studies by Ochoa-Fuentes et al. (2015) and Belisle et al. (2019), who also observed variability in the virulence of P. cinnamomi isolates infecting avocado plants.
Phytophthora cinnamomi can infect plants at various developmental stages. This microorganism causes damage to the roots and triggers the appearance of various symptoms such as yellowing, leaf wilting, defoliation, regressive branch death, and ultimately, tree decline (Castaño & Leal, 2018). The presence of P. cinnamomi affecting avocado plants has been extensively documented worldwide (EPPO, 2004; Miyambo et al., 2022). In Colombia, its presence has also been confirmed in various departments, including Antioquia, Cauca, Risaralda, Tolima, and Valle del Cauca (Ramírez-Gil, 2018; Marulanda, 2018).
Phytophthora cinnamomi is considered as the most important species within the avocado wilt complex due to its frequency, aggressiveness, and the considerable losses it causes (Miyambo et al., 2022). This study confirmed the high degree of virulence exhibited by P. cinnamomi in avocado seedlings. Several research works have highlighted its ability to infect and cause the death of avocado plants rapidly, characteristics associated with factors such as its speed in penetrating and colonizing plant tissue (Castaño & Leal, 2018).
Phytophthora heveae has been documented in Guatemala, where it was identified as the causal agent for avocado stem canker (Zentmyer et al., 1978). Later, its presence was reported in Mexico, showing similar symptoms of stem canker (Ceja-Torres et al., 2000). Ochoa-Ascencio et al. (2011) identified P. heveae as the cause of avocado fruit rot in Mexico. In Colombia, there have been no reports of this pathogen causing root rot in avocado seedlings in nurseries. However, in 2014, its presence was reported in the Antioquia department, where it induced cankers at the base of the stems of mature avocado trees in the field (Ramírez-Gil et al., 2014).
This research makes a significant contribution to the advancing scientific knowledge by identifying pathogenic agents associated with avocado root rot disease in the nursery phase.
4. Conclusions
Phytophthora cinnamomi and P. heveae were identified through morphological, molecular, and pathogenic analyses, confirming their role as causative agents of wilting and root rot in avocado plants in nursery material. Both species demonstrated a high pathogenic capacity, leading to symptoms such as leaf wilting, chlorosis, progressive wilting, and plant death. The presence of P. cinnamomi and P. heveae poses a significant risk during the crop establishment phase.
This work serves as the foundation for the develop-ment and implementation of comprehensive disease management strategies, as well as the selection of pathogen-resistant rootstocks.
Acknowledgments
The authors express their gratitude to the ICA (Colombian Agricultural Institute) registered nurseries in the departments of Quindío, Risaralda, and Valle del Cauca for providing avocado plants for pathogen diagnosis. To Dr. Takumasa Kondo (AGROSAVIA) to review an early version of the text. Special thanks to the Corporación Colombiana de Investigación Agropecuaria - AGROSAVIA and the Ministry of Agriculture and Rural Development of Colombia for funding this research. The study is part of the project "Technological foundations for the production of high-quality avocado planting material in producing nurseries - ID 1001719".
Author contributions
L. P. Palacios-Joya: Methodology, Investigation, Formal analysis, Writing – original draft, Writing – review & editing. K. A. Rodríguez-Arévalo: Methodology, Investigation, Formal analysis. Writing – review & editing. M. F. Martínez: Investigation, Resources, Funding acquisition, Project administration, Writing – review & editing. N. Murcia-Riaño: Methodology, Resources, Supervision, Writing – review & editing. D. M. Rodríguez-Mora: Methodology, Investigation, Formal analysis, Supervision, Writing – original draft, Writing – review & editing.
Declaration of interest statement
The authors declare no affiliation or involvement with any entity that could have financial or equity interest that jeopardizes the validity of the presented results. The funders were not involved in the design of the study, methodology, data analysis, results interpretation or writing of the manuscript.
ORCID
L. P. Palacios-Joya https://orcid.org/0000-0003-2201-1432
K. A. Rodríguez-Arévalo https://orcid.org/0000-0002-1058-8464
M. F. Martínez https://orcid.org/0000-0002-8145-1764
N. Murcia-Riaño https://orcid.org/0000-0002-5679-3885
D. M. Rodríguez-Mora https://orcid.org/0000-0001-6545-3746
References
Abad, Z. G., Burgess, T., Redford, A. J., Bienapfl, J. C., Mathew, R., et al., (2023). IDphy: An international online resource for molecular and morphological identification of Phytophthora. Plant Disease, 107(4), 987–998. https://doi.org/10.1094/pdis-02-22-0448-fe
Agapito, E. M., Cibrián-Llanderal, V. D., Gutiérrez, M., Ruiz-Juárez, D., López-Corona, B. E. et al. (2022). Phytophthora cinnamomi Rands in avocado. Revista Mexicana de Ciencias Agrícolas, 13(28), 331–341. https://doi.org/10.29312/remexca.v13i28.3287
Álvarez, A., Oliveros, D., Ávila, Y. C., Sabogal-Palma, A. C., Murillo, W., et al. (2023). Resistance induction with silicon in Hass avocado plants inoculated with Phytophthora cinnamomi Rands. Plant Signaling & Behavior, 18(1), 1-11. https://doi.org/10.1080/15592324.2023.2178362
Ángel-García, C.; Rodríguez-Arevalo, K. A.; Murcia Riaño, N.; Martínez-Caballero, L. N.; Ceballos-Aguirre, G., et al. (2023). Molecular identification and fungal diversity associated with diseases in Hass avocado fruit grown in Cauca, Colombia. Pathogens, 12(12), 1418. https://doi.org/10.3390/pathogens12121418
Belisle, R. J., Mckee, B., Hao, W., Crowley, M., Arpaia, M. L., et al. (2019). Phenotypic characterization of genetically distinct Phytophthora cinnamomi isolates from avocado. Phytopathology, 109(3), 384–394. https://doi.org/10.1094/PHYTO-09-17-0326-R
Castaño, Z. J., & Leal, J. M. (2018). Manejo integrado de la pudrición de raíces del aguacate (Persea americana Miller), causada por Phytophthora cinnamomi Rands. Temas Agrarios, 23(2), 131-143. https://doi.org/10.21897/rta.v23i2.1297
Ceja-Torres, L. F., Téliz-Ortiz, D., Osada-Kawasoe, S., & Morales-García, J. L. (2000). Etiología, distribución e incidencia del Cancro del aguacate Persea americana Mill. en cuatro municipios del Estado de Michoacán, México. Revista Mexicana de Fitopatología, 18, 79-86.
Dixon, K. W. T., & Sivasithamparam, K. (1984). Technique for rapid assessment of tolerance of Banksia spp. to root rot caused by Phytophthora cinnamomi. Plant Disease, 68, 1077-1080.
European and Mediterranean Plant Protection Organization (EPPO). (2004). Phytophthora cinnamomi. Diagnostic protocols for regulated pests. European and Mediterranean Plant Protection Organization. Bulletin. https://doi.org/10.1111/j.1365-2338.2004.00720.x. Access on: April 28, 2024.
FAOSTAT. Crops and livestock products. Available at: < https://www.fao.org/faostat/en/#data/QCL >. Accessed on: Feb. 23, 2024.
Lanete, P. J. C., Tendenilla, S. L. S., Trinidad, M. S. L. R., & Bennett, R. M. (2023). An insight into the genus Phytophthora (Peronosporaceae: Oomycota). Philippine Journal of Systematic Biology, 17(1),9-15.
Leal, J., Castaño-Zapata, J., & Bolaños, M. (2014). Manejo de la Pudrición radical (Phytophthora cinnamomi Rands) del aguacate (Persea americana Linneo). Revista U.D.C.A Actualidad & Divulgación Científica, 17(1),105-114. https://doi.org/10.31910/rudca.v17.n1.2014.945
Marulanda, C. (2018). Identificación de aislamientos de Phytophthora cinnamomi asociados al cultivo de aguacate en el sur occidente de Colombia. Thesis (M.sc) - Facultad de Ciencias Agropecuaria, Universidad Nacional de Colombia, Palmira.
Miyambo, T. M., Backer, R., Engelbrecht, J., Joubert, F., & Van Der Merwe, N. A., (2022). The identification and characterization of endopolygalacturonases in a South African isolate of Phytophthora cinnamomi. Microorganisms, 10(5), 1061. https://doi.org/10.3390/microorganisms10051061
Kavroulakis, N., Tziros, G., Mikalef, L., & Malandrakis, A. (2024). First Report of Phytophthora cinnamomi causing root rot of avocado trees in Greece. Plant Disease. doi: 10.1094/PDIS-05-24-0939-PDN
Ochoa-Ascencio, S., Santacruz-Ulibarri, H., & Salazar-García, S. (2011). Phytophthora heveae causing basal rot of avocado fruit in México. En: VII World Avocado Congress, Cairns, Australia, Proceedings.
Ochoa-Fuentes, Y. M., Cerna-Chávez, E., Gallegos-Morales, G., Cepeda-Siller, M., Landeros-Flores, J., et al. (2015). Variabilidad patogénica de Phytophthora cinnamomi Rands en Persea americana Mill. de Michoacán, México. Ecosistemas y recursos agropecuarios, 2(5), 211-215.
Parkinson, L. E., Shivas, R. G., & Dann, E. K. (2017). Pathogenicity of nectriaceous fungi on avocado in Australia. Phytopathology, 107(12), 1479–1485. https://doi.org/10.1094/PHYTO-03-17-0084-R
Raeder, U., & Broda, P. (1985). Rapid preparation of DNA from filamentous fungi. Letters in Applied Microbiology, 1, 17–20. https://doi.org/10.1111/j.1472-765X.1985.tb01479.x
Ramírez-Gil, J., Castañeda, D., & Morales, O. (2014). Estudios etiológicos de la marchitez del aguacate en Antioquia-Colombia. Revista Ceres, 61(1), 50–61. https://doi.org/10.1590/S0034-737X2014000100007
Ramírez-Gil, J., Gilchrist, R., & Morales, O. (2017). Economic impact of the avocado (cv. Hass) wilt disease complex in Antioquia, Colombia, crops under different technological management levels. Crop Protection, 101, 103–115. https://doi.org/10.1016/j.cropro.2017.07.023
Ramírez-Gil, J. (2018). Avocado wilt complex disease, implications and management in Colombia. Revista Facultad Nacional de Agronomía Medellín, 71(2), 8525–8541. https://doi.org/10.15446/rfna.v71n2.66465
Ramírez-Gil, J., & Morales, O. (2020). Development and validation of severity scales of avocado wilt complex caused by Phytophthora cinnamomi, Verticillium dahliae and hypoxia-anoxia disorder and their physiological responses in avocado plants. Agronomía Colombiana, 38(1), 85-100. https://doi.org/10.15446/agron.colomb.v38n1.78527
Saltarén, L. F., Varón De Agudelo, F., & Marmolejo, F. (1998). Patógenos radicales en material de propagación de aguacate (Persea americana Mill.). Fitopatología Colombiana, 22(2), 52-58.
Tsao, P. H., & Guy, S. (1983). Factors affecting isolation and quantitation of Phytophthora from soil. In Erwin, C., Barnicki-Garcia, S., Tsao, P. H. (Eds.) Phytophthora: Its Biology, Taxonomy, Ecology and Pathology (pp. 219-235). American Phytopathological Society Press.
Villarino, M., Larena, I., Melgarejo P., & De Cal, A. (2021). Effect of chemical alternatives to methyl bromide on soil‐borne disease incidence and fungal populations in Spanish strawberry nurseries: A long‐term study. Pest Management Science, 77(2),766-774. https://doi.org/10.1002/ps.6077
White, T. J., Burns, T., Lee, S., & Taylos, J. (1990). Amplification and Direct Sequencing of Fungal Ribosomal RNA Genes for Phylogenetics. In M. Innis, D. Gelfand, J. Sninsky, & T. White (Eds.), PCR Protocols: A Guide to Methods and Applications (pp. 315–322). Academic Press.
Zentmyer, G. A., Klure, L. J., & Pond, E. C. (1978). A new canker disease of avocado caused by Phytophthora heveae. Plant Disease Reporter, 62, 918-922.