REVIEW
Advances in the use of active yeast in raising chickens
Gabriel Aguirre-Guzmán1 * ; José Octavio Merino-Charrez1 ; María Lorena Torres-Rodríguez1 ; Miguel Angel Guevara-Guerrero1
1 Universidad Autónoma de Tamaulipas, Facultad de Medicina Veterinaria y Zootecnia, km 5 Carretera Victoria, Mante, Ciudad Victoria, Tamaulipas, México.
* Corresponding author: gabaguirre@docentes.uat.edu.mx (G. Aguirre-Guzmán).
Received: 8 July 2024. Accepted: 31 December 2024. Published: 18 January 2025.
Abstract
The broiler industry supplies quality protein, which is in constant development. It seeks productive strategies that improve production, health, growth, and survival and reduce the poultry industry's diseases, stress, and long-term environmental impact. Broiler chickens are exposed to numerous microorganisms that alter production, and this is an opportunity for yeasts to promote the growth of organisms, stimulate the immune system, improve health, promote changes in intestinal structure, and inhibit pathogens. This review summarizes the current knowledge and effect of active yeast species on raising chickens, nutrition, immunity, digestibility, changes in intestinal structure, and pathogens on those organisms. Due to their nutritional value, active yeasts are used as natural and alternative ingredients in broiler chickens. They are a source of b-glucans, chitin, nucleic acids, mannan-oligosaccharides, b-carotene, and vitamins. Enzymes they produce improve intestinal maturity and digestion. The immune and antioxidant properties of yeasts play an essential role as probiotics and immunostimulants to enhance the resistance of broilers against common viral and bacterial diseases. Bioactive products generated by active yeasts can improve intestinal microbiota and positively alter the immune response, phagocytosis, encapsulation, etc. Different active yeast species and strains have been used and have generated exciting results. They are popular as beneficial candidates for nutrition by maintaining broiler chickens’ health's and well-being conditions. Future studies must understand the functioning and effect of species and strains on broiler chickens in their different processes, the use of new research tools (proteomics, radioisotopes, real-time molecular biology, etc.) can facilitate these studies.
Keywords: broiler environment; poultry industry; raising conditions; yeast; animal welfare.
DOI: https://doi.org/10.17268/sci.agropecu.2025.009
Cite this article:
Aguirre-Guzmán, G., Merino-Charrez, J. O., Torres-Rodríguez, M. L., & Guevara-Guerrero, M. A. (2025). Advances in the use of active yeast in raising chickens. Scientia Agropecuaria, 16(1), 93-111.
1. Introduction
Modern broiler breeds are the result of artificial selection for commercial purposes. Broilers, in different breeds, are an essential source of high-quality protein for consumption that is constantly growing worldwide (Dixon et al., 2022). The size of broilers for consumption (4 or more weeks) may vary depending on the breed and production area (Asia, Europe, and North America). However, the industry is constantly affected by infectious diseases (viruses and bacteria) that generate significant losses in production (Bagust, 2013; Dixon et al., 2022; FAO, 2023).
The rapid and uncontrolled growth of pathogens in broiler chickens and the excessive use of antibiotics as prophylactics have generated the emergence of several resistant pathogens that cause acute and chronic diseases that reduce the development and sustainability of the industry worldwide (Haldar et al., 2011; Fanelli et al., 2015; Ahiwe et al., 2021). Antibiotics improve food safety and animal health by reducing or eliminating pathogens. However, due to the side effects of antibiotics (accumulation, resistance, etc.), these products become a threat that must be eliminated, reduced, or replaced with new products or alternative strategies that benefit production and the environment (Haldar et al., 2011; Ahiwe et al., 2021; Fathima et al., 2023). Alternative methods of disease prevention have been designed via antioxidant systems, bioremediation, genetics, immunostimulants, nutrition, probiotics, prebiotics, symbiotics, etc. (Haldar et al., 2011; Khan & Naz, 2013; Sapsuha et al., 2021).
Yeast is one of the first domesticated organisms, of which 1% of the yeast species are known (Fell, 2001; Patterson et al., 2023). These have demonstrated the potential to produce bioactive products (glucans, glutathione, enzymes, phytase, vitamins, etc.) with application in the chemical, cosmetic, food, pharmaceutical, animal protection, and reduction of adverse environmental effects industries (Cheng et al., 2014; Zaky et al., 2014; Sarkar & Bhaskara, 2016; Fathima et al., 2023; Patterson et al., 2023). Yeast (whole, parts, molecules, extracts) are a popular product in animal production as a food supplement, source of amino acids, and proteins. They participate in the production of B complex vitamins, digestive enzymes, stimulation of the immunity of the intestinal mucosa, greater protection against toxins produced by pathogenic microorganisms, and a greater number of anaerobic bacteria that reduce harmful gases. These generate positive effects on the growth and immunity of the broiler chicken (Saied et al., 2011; Adebiyi et al., 2012).
Active or living yeasts, like other cells, have a complex biochemical system where different internal metabolic systems interact. It is necessary to document its effect on monogastric better (Gao et al., 2008), its relationship with systems related to dispersion mechanisms, the interaction between cells (inter or intraspecies), cellular communication, and the production of extracellular molecules in response to chemical and physical stimuli from the external environment and the environment, inside the cell. Cells also have multiple metabolic mechanisms for defense, adhesion, colonization, etc. This review summarizes the current knowledge of the main yeast strains used as probiotics, feed additives, and immunostimulants to reduce feed costs and improve growth and survival in broiler production. The information provided is focused on works that directly used active yeast, showing its effects on digestion, growth parameters, immune system, meat quality, metabolism, and physiology.
2. Yeast used in broilers chicken
Broiler chickens interact with a high and diverse range of environmental and microorganisms, which could play a vital role in their growth, survival, and welfare. Yeasts are microorganisms usually found in the environment and areas and organisms under raising, documenting their role in the health and nutrition of broiler chickens. These microorganisms have been used live (active) as ingredients or food supplements to feed broilers, generating multiple beneficial effects. Some yeast genera (Candida sp., Cyberlindnera sp., Debaryomyces sp., Kluyveromyces sp., Phaffia sp., Rhodotorula sp., Torulaspora sp., Saccharomyces sp., Yarrowia sp., and Zygosaccharomyces) are part of the intestinal flora of the broiler and have shown their application in the cultivation of this organism (in their different breeds)(Gao et al., 2008; Haldar et al. 2011; Cafarchia et al., 2018; Laubscher et al., 2020; Ahiwe et al., 2021; Bilal et al., 2021; Grabež et al., 2022; Kim et al., 2022; Liu et al., 2022; Dedousi et al., 2023). A better understanding of the recent use and modes of action of active yeast in broilers is present in Tables 1-5.
2.1. Candida. This group of yeasts is from the class Saccharomycetes, division Ascomycota, and at least 100 species are described. This yeast has about 200 species, many of which are commensals, harmless endosymbionts, or opportunistic pathogens. This yeast is part of the bacterial flora on the surface of the mucous membranes, mainly those of the gastrointestinal tract or the skin. They are widely distributed in nature and can grow in different environments and temperatures. Yeast cells are globose, ellipsoidal, cylindrical, or elongated in shape and occasionally ogival and triangular during asexual reproduction (Hommel, 2014). The Candida yeast that has been used as an ingredient (active cell) in research works associated with broiler chickens is Candida utilis (Rodríguez et al., 2013; Wang et al., 2020) (Table 1, 3, 4).
2.2. Cyberlindnera. Previously called Lindnera, it shows an asexual reproduction, budding is multilateral on a narrow base, and their cells are spherical, oval, or elongated. In sexual reproduction, asci may be conjugated, showing conjugation between cells. Also, some species are heterothallic (Kurtzman, 2011). This author shows that there are several species of this yeast (Cyberlindnera americana, Cy. amylophila, Cy. bimundalis, Cy. euphorbiae, Cy. euphorbiiphila, C. fabianii, C. jadinii, Cy. japónica, etc.).
2.3. Debaryomyces. They are from the class Saccharomyces, division Ascomycota, they are commonly found in the environment, they are widely used in the food industry, and at least 30 species have been described. Their cells are globose, ovoid, or lenticular in shape during asexual reproduction, which is of the multilateral germination type (Wrent et al., 2014). Genetic studies show different defined species; however, some of them have high genetic heterogeneity (Debaryomyces artagaveytiae, D. carsonii, D. castellii, D. coudertii, D. etchellsii, D. globularis, D. hansenii, etc.)(Wrent et al., 2014).
Table 1
Effect of active yeast on growth parameters of broilers chicken
Yeast | Broilers chicks | Doses | Use | References |
Candida utilis | Cornish x White Plymouth Rock, hybrid HE21 (both sexes, 1-day-old) | 10-30% | The 30% dose shows a significantly higher value is observed in total feed intake (FI), feed conversion ratio (FCR), and lower live weight, and live weight gain. | Rodríguez et al., 2013 |
Debaryomyces hansenii | Ross 308 (male and female 1-day-old) | 5×106 CFU kg feed-1 | Body weight, feed consumption (FC), weight gain, and FCR were similar controls. | Liu et al., 2022 |
Kluyveromyces marxianus | Arbor Acre (1-day-old female) | 0.25-2.5 g kg-1 (2.0×1010 CFU g-1) | A significant reduction in the FCR compared to the control (2-2.5 g kg-1). | Wang et al., 2017a |
K. marxianus | Ross-308 (1-day-old) | (4.125×106 CFU 100 mL-1) | Significant increase in body weight, lower FC and better FCR | Khalifa et al., 2024 |
K. marxianus | Ross308 (1-day-old) | 0.002-0.005% | Significantly reduces mortality | Rassmidatta et al., 2024 |
Phaffia rhodozyma | Ross broilers (14-day-old female) | 10-20 mg kg-1 | Higher body weight gain, feed intake, and feed efficiency compared to the control group. | Perenlei et al., 2014 |
Saccharomyces boulardii | Sanhuang (200-day-old) | 108 CFU kg-1 | Significant increase in body weight compared to the control group. | Sun et al., 2017 |
S. cerevisiae | Broiler chick (180-day-old) | 0.2% | Significant increase in body weight. | Churchill et al., 2000 |
S. cerevisiae | Ross breed (male, 1-day-old) | 0.5% | Used in growth parameter generating a gain in body weight compared to control. | Zhang et al., 2005 |
S. cerevisiae | Ross (male 240-day-old) | 0.5-2% | The use of 1.5% yeast in the feed significantly increases body weight gain and FI and shows a significantly better FCR. | Paryad & Mahmoudi, 2008 |
S. cerevisiae | Arbor Acres (male, 1-day-old) | 2.5 g kg-1 diet | Significant increase in average daily weight gain and FCR. Significantly increased the digestibility of calcium and phosphorus but did not affect protein and energy retention digestibility. | Gao et al., 2008 |
S. cerevisiae | Ross 308 | 0.5-2% (8×109 CFU g-1) | Significant results in FI, live weight, and final weight gain in organisms fed yeast diets. | Hosseini, 2011 |
S. cerevisiae | Cobb (1-day-old) | 1 g kg-1 diet | Significantly improves final weight, daily and total weight gain, and FCR. | Haldar et al. 2011 |
S. cerevisiae | Cobb (1-day-old, 45 g) | 0.5 g Live yeast kg diet-1 | Significant decrease in FI, FCR, and mortality. Significant increase in final post-slaughter weight. | Iraqi & Fayed, 2012 |
S. cerevisiae | hybrid Hubbard (1-day-old) | 0.25 mL (4.12×106 CFU 100 mL-1) | Significantly positive effect on body weight gain at 4th week of age compared with the control. | Aluwong et al., 2013 |
S. cerevisiae | Arbor Acres (1-day-old, 40 g) | 3% | Significantly improved body weight gain and FCR. | Tabidi et al., 2013 |
Saccharomyces cerevisiae | Hubbard (1-day-old, 45 g) | 0-3% | Better values in final body weight, weight gain, FI, FCR, and mortality. | Eltazi et al., 2014 |
S. cerevisiae | Aber acer (unsexed 1-day-old, 45 g) | 0.1-0.3% | 0.3% use of dry yeast had significantly higher body weight gain and the best FCR compared to control. | Hana et al., 2015 |
S. cerevisiae | Arbor Acres (1-day-old male) | 0.5 g kg-1 (1.0 × 1010 CFU g-1) | Significantly reduces the FCR. | Wang et al., 2016b |
S. cerevisiae | Cobb 400 (288-day-old) | 0.1-0.2% | Significant values in body weight gain, and feed efficiency. | Shankar et al., 2017 |
S. cerevisiae | Cobb 500 (unsexed, 165-day-old) | 0.5-3% | A positive significant difference is observed in the total weight gain and the FCR. | Lawrence-Azua et al., 2018 |
S. cerevisiae | Cobb (1-day-old, 43 g) | 0.2% | Significantly improved live body weight, live body weight gain, and FCR. | Mousa, 2018 |
S. cerevisiae | Cobb (42 g) | 2.5% | Significant values in final weight, total weight gain, daily weight gain, FCR, FC, and FI compared to control. | Mulatu et al., 2019 |
S. cerevisiae | Arbor Acres (1-day-old male, 45.2 ± 0.46 g) | 0.5-1 g kg−1 (1010 CFU g−1) | A significant increase in final body weight and mean daily weight gain is observed at the highest dose of yeast. | He et al., 2022 |
S. cerevisiae | Hubbard (1-day-old) | 1.5 g kg-1 | Weight gain, FC, and FCR improved significantly with a diet containing yeast. | Rafique et al., 2020 |
S. cerevisiae | Arbor Acres (unsexed 1-day-old) | 0.04% kg feed-1 (1.2×1010 active yeast g-1) | Significant improvement in growth rate and digestibility of organic and apparent matter detected in the ashes. | Attia et al., 2020 |
S. cerevisiae | Broiler chicks (4 we old 0.89 kg) | 0.7-1.7 g kg of basal diet-1 | Significant values in final body weight, total body weight gain, daily weight gain, and FCR. | Osita et al., 2020 |
S. cerevisiae | Arbor Acres (male 1-day-old, 45.23 g) | 1 g kg-1 (1010 CFU g-1) | The final body weight was significantly higher compared to the control. | He et al., 2021 |
S. cerevisiae | Cobb 500 (male 1-day-old) | 0.1% (1010 live yeast cells, 52 g) | A significant difference is observed in final body weight, body weight gain, FI, and FCR. | El-Manawey et al., 2021 |
S. cerevisiae | Mixed-sex (45.10 g) | 1 g kg-1 diet | Significantly increased daily weight. | Kim et al., 2022 |
S. cerevisiae | Broiler chicks | 2% | Significant result in FI, and body weight. | Gul & Alsayeqh, 2023 |
2.4. Kluyveromyces. This is a member of the ascomycetous family Saccharomycetaceae. The molecular studies revealed there are approximately six species (Kluyveromyces aestuarii, K. drosophilarum, K. lactis, K. marxianus, K. nonfermentans, and K. wickerhamii). It has a worldwide distribution and grows in different environments, this changes its morphological, physiological, and molecular characteristics, which complicates its identification. The best-known species in the industry are K. lactis and K. marxianus for their relationship in the ripening of cheeses, yogurt, etc. It is an attractive commercial yeast species because it has higher growth rate, metabolic functions, and high mannan content in the cell wall than Saccharomyces cerevisiae (Belloch et al., 2011; Wang et al., 2017a).
2.5. Phaffia. This is an orange color Deutero-mycotina yeast (Blastomycetes) genus. It ferments glucose and other sugars to produce carotenoids that give color to the yeast, with astaxanthin being the main carotenoid produced. The yeasts have a red-orange color in their basidia. This yeast and its pigments are used as an additive in animal feed to enhance the color of the eggs and meat of terrestrial and aquatic organisms, and they are also important natural antioxidants. The following species are known: Phaffia aurantiaca, P. australis, P. brasiliana, P. rhodozyma, and P. tasmanica. Some recent publications indicate P. rhodozyma as a yeast species with the potential to be used generating interesting results in growth (Perenlei et al., 2014).
2.6. Saccharomyces. It is the most widely used yeast in broilers (Table 1-5), it is also the most widely used yeast in different biotechnological and fermentation processes worldwide, such as the production of beer, wine, and bread, and it is the first eukaryotic cell whose genome was sequenced (Alsammar & Delneri, 2020; Ahiwe et al., 2021). They are from the class Saccharomyces, division Ascomycota, which reproduces by budding, presenting a haploid and diploid life cycle. Genetic studies indicate the existence of eight well-defined species (Saccha-romyces arboricolo, S. cerevisiae, S. eudayanus, S. jurei, S. kudiavzevii, S. mikatae, S. paradonus, and S. uvarum) (Alsammar & Delneri, 2020).
2.7. Yarrowia. They are from the class Saccharomyces, division Ascomycota, which has asexual growth by multilateral budding. There is only the species Yarrowia lipolytica; its cells have a spherical, ellipsoidal, or elongated shape, found alone, in pairs, or in small groups. Also, it has dimorphic characteristics, pseudomycelium, and branching can be observed (Sutherland et al., 2014). Patsios et al. (2020) show that Y. lipolytica is detected in food products such as meat, fish, dairy products, etc., and can grow in different agricultural by-products with high levels of fatty acids. This yeast is used in terrestrial and aquatic organisms, generating positive effects on their nutrition since it stores essential fatty acids intracellularly or lipid bodies (≤ 20%) and has a low content of nucleic acids that increases the palatability of diets. It also secretes heterologous proteins, has a high lysine content, generates enzymes that increase animal digestion (esterases, lipases, phosphatases, and proteases), and is a large producer of citric acid (Mirbagheri et al., 2012; Patsios et al. 2020; Guardiola et al., 2021).
3. Yeast effect on broilers chicken
The increase in the consumption of products of animal origin is estimated in the following decades, with an annual consumption of 25.5 to 37 kg per person in developed countries and 88 to 100 kg per person in industrialized countries by 2030 (FAO, 2003; Patsios et al., 2020). The livestock sector is constantly growing because of population growth, economic income, and lifestyle and dietary habits changes. Satisfying this constant growth and maintaining the natural resource base (soil, water, air, and biodiversity) are significant challenges facing global livestock today. Poultry production is significant due to the short time in its production cycle, the quality of its meat, and low cost for the consumer. This increase in production has been strengthened by moving from extensive to intensive integrated activity (market, consumers, cost). This phenomenon has increased competition for ingredients for poultry nutrition, increasing the environmental and public health risks of this industry as it is located near urban areas (FAO, 2003, 2023). Feed represents a high cost in the production of broiler chickens (>50%), and efficiency in the use of ingredients and additives that favor its use is significant in production (FAO, 2003). Yeasts are microorganisms that have been used as animal feed supplements. Its application is greatly facilitated by its high acceptability and availability; several species and/or yeast strains are native to various food products (meat, fish, dairy products, etc.) (Patsios et al., 2020).
Figure 1 shows the main areas of study where the studies evaluated in the present research (2000-2023) have focused on the use of active yeasts in broiler chicken, where the areas of growth parameters (weigh, feed conversion rate, survival, feed intake, etc.), blood parameters (cell number, immunology, immunostimulant, blood chemistry, etc.), microorganisms present in broilers, size of chicken organs and tissues, intestine characteristics, and behavior of the birds were the most studied.
3.1. Yeast effect on growth parameters of broilers chicken
Growth parameters are an important indicator of the effectiveness of the application of an ingredient in the diet of organisms and are a starting point to quantify the future environmental impact of the poultry sector (Cheng et al., 2014). The growth of organisms is directly related to the balance of nutrients in the diet and the ingredients that make it up. Protein is the most expensive and limiting nutrient in the production of animal feed, The decrease in this nutrient or its quality results in poor growth, diseases, etc. (Adebiyi et al., 2012; Rafique et al., 2020; Patterson et al., 2023).
The use of alternative protein ingredients or supplements is necessary to strengthen the animal feed supply chain, also reducing the impact on the environment. This has allowed the use of new protein sources (insect meal, micro and macro-algae, microorganisms, byproducts of the food industry, etc.) in animal feed (Patterson et al., 2023).
One of the poultry industry's challenges is maintaining productive efficiency through the proper use of feed and its ingredients (Paryad & Mahmoudi, 2008). In recent decades, the production of some traditional ingredients used in animal feed has decreased, allowing the use of alternative ingredients as possible substitutes. Yeast has been used as an ingredient in the diets of horses, cows, aquatic organisms, etc., and is successfully used in poultry feed as a partial replacement for the traditional protein source (Rameshwari & Karthikeyan, 2005; Rodríguez et al., 2013). Yeasts have been used in diets for broiler breeding due to their nutritional value (proteins, lipids, vitamins, minerals, etc.) and some bioactive compounds (amino acids, β-glucans, chitin, oligosaccharides, nucleic acids, etc.) promoting feed efficiency and improving overall performance (Paryad & Mahmoudi, 2008; Morales-López et al., 2009; Saied et al., 2011; Bilal et al., 2021). Different types of active yeasts (Table 1) and their extracts have been used in broiler-raising diets at different levels (0.1 - 30% or 0.1 - 5 g kg-1).
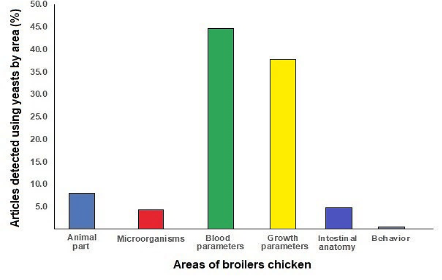
Figure 1. The main areas of study in the articles detected the use of active yeast in broilers chicken from 2000-2024.
In addition, Figure 2A shows the subject of studies associated with the area of growth parameters, where 50% of the works focused on evaluating growth, 20% on conversion factor (FCR), 16% on consumption, 7% on digestibility, and almost 3% on mortality.
Rodríguez et al. (2013) and Perenlei et al. (2014) used Candida utilis and Phaffia rhodozyma at different doses, detecting significant improvements in live weight, weight gain, FCR, and feed intake at the highest dose. Wang et al. (2017a) and Liu et al. (2022) used Kluyveromyces marxianus and Debaryomyces hansenii at different doses, improving the feed conversion ratio, respectively. Table 1 shows that Saccharomyces cerevisiae is the most widely used yeast in diets as a growth promoter for broilers, which has been used in different broiler breeds and hybrids by supplying it in their feed or drinking water. This yeast has been applied at doses of 0.04 - 3%, 0.5 - 2.5 g kg-1 diet, and 106 - 1010 CFU g-1 with positive and significant effects on different growth parameters. Haldar et al. (2011) and Mousa (2018) show average daily gain and FCR when supplying S. cerevisiae (1 g kg-1) or 0.2% at 35 d post-application, respectively. Gao et al. (2008) used active S. cerevisiae yeast at a dose of 2.5 g kg-1 diet, significantly improving daily weight gain, and FCR: increasing the digestibility of calcium and phosphorus. Similar results were obtained by Hana et al. (2015), El-Manawey et al. (2021) and He et al. (2021). Attia et al. (2020) obtained similar results with the digestibility of organic and apparent matter detected in the ashes. Sun et al. (2017) and Bilal et al. (2021) used S. boulardii, revealing significant results in body weight gain, particularly during the early life of broiler chickens. Iraqi & Fayed (2012), Aluwong et al. (2013), Tabidi et al. (2013), Eltazi et al. (2014), and Lawrence-Azua et al. (2018) used active S. cerevisiae yeast and improving weight gain, FCR, and/or reducing mortality of the organisms.
Table 4
Effect of active yeast on microorganisms of broilers chicken
Yeast | Broilers chicks | Doses | Use | References |
Debaryomyces hansenii | Ross 308 (male and female 1-day-old) | 5×106 CFU kg feed-1 | Significantly increase the presence of lactic acid bacteria in the cecum. | Liu et al., 2022 |
Kluyveromyces marxianus | Arbor Acre (1-day-old female) | 0.25-2.5 g kg-1 (2.0×1010 CFU g-1) | The abundance of Cyanobacteria, Rickettsiales, Pseudomonadales, and Acinetobacter junii decreased. There is also an increased abundance of Firmicutes and Lactobacillus sp in the ileum. | Wang et al., 2017a |
Saccharomyces cerevisiae | Cobb (1-day-old) | 1 g kg-1 diet | Significantly lower levels of Salmonella enteritidis are observed in the digesta and feces, and lower in blood (35 d post-challenge, oral application at 108 CFU mL-1). Significantly lower levels of Escherichia coli are observed in the digest, and lower in blood (same condition). | Haldar et al. 2011 |
S. cerevisiae | Hubbard (female, 1-day-old) | 106 CFU g-1 feed | Significantly decreased the presence of Salmonella sp and Campylobacter sp on the neck, breast, cecum, and feces. | Fanelli et al., 2015 |
S. cerevisiae | Both sex (45.10 g) | 1 g kg-1 diet | Lower diversity of microbiota in the ileal digest (inverse Simpson diversity), with an increase in the abundance of the Firmicutes phylum, and genus Lactobacillus, Prevotella, and Enterococcus compared to the control. | Kim et al., 2022 |
Table 5
Effect of active yeast on intestinal anatomy of broilers chicken
Yeast | Broilers chicks | Doses | Use | References |
Kluyveromyces marxianus | Arbor Acre (1-day-old female) | 0.25-2.5 g kg-1 (2.0×1010 CFU g-1) | Increase in the ratio of villus height to crypt depth of the jejunum and ileum, in addition to ileal villus height and sucrase activity, and mRNA expression in the ileum for mucin-2 and sodium-glucose. | Wang et al., 2017a |
K. marxianus | Ross308 (1-day-old) | 0.002-0.005% | Enhanced the villus height/crypt depth ratio compared to control | Rassmidatta et al., 2024 |
Saccharomyces boulardii | Sanhuang (200-day-old) | 108 CFU kg-1 | A greater height of the villus and crypt of duodenum compared to control | Sun et al., 2017 |
S. cerevisiae | Male broilers (Ross breed, 1-day-old) | 0.5% | A greater height of the villus (ileal mucosa) is shown in comparison with the control. | Zhang et al., 2005 |
S. cerevisiae | Arbor Acres (male, 1-day-old) | 2.5 g kg-1 diet | Significant increase in the height of the villus and depth of crypts in the duodenum, jejunum, and ileum. | Gao et al., 2008 |
S. cerevisiae | Cobb (1-day-old) | 1 g kg-1 | Significant increase in the height of the villus in the duodenum, and significantly decreased crypt, and serosa depth in the ileum. | Haldar et al. 2011 |
S. cerevisiae | Arbor Acres (1-day-old male) | 0.5 g kg-1 (1010 CFU g-1) | Significant increase in villus height and the ratio of villus height to ileal crypt depth. | Wang et al., 2016b |
S. cerevisiae | Cobb (1-day-old male) | 0.05-0.5% (1010 CFU g−1) | Increase the width of the jejunal, ileal microvilli, surface area of the microvilli (21 d), and villus height to crypt depth ratio, and reduce jejunal maltase activity. | Wang et al., 2017b |
S. cerevisiae | Arbor Acres (unsexed 1-day-old) | 0.02 and 0.04% kg feed-1, 1.2×1010 active yeast g-1) | Significant effect on the length of the microvilli. | Attia et al., 2020 |
S. cerevisiae | Cobb 500 (45-day-old) | 5 mg kg diet-1 (107 CFU g-1) | The significantly greater area of the crypts of duodenum and jejunum, a smaller number of crypts mm-1 in the duodenum, and higher mucus production in the same tissue compared to the control | Quevedo et al., 2020 |
S. cerevisiae | Arbor Acres (male 1-day-old, 45.23 g) | 0.5-1 g kg-1 (1010 CFU g-1) | The height in the microvilli of the jejunum and ileum was significantly higher compared to the control. | He et al., 2021 |
S. cerevisiae | Both-sex (45.10 g) | 1 g kg-1 diet | Increase in the height and area of the duodenal microvilli and reduction in the depth of the duodenal crypts compared to the control | Kim et al., 2022 |
3.8. Effect of yeast on the microbiota of the digestive system
The health and growth of broilers are associated with intestinal health and microflora, this helps against pathogens and assimilates more nutrients, which promotes animal well-being and development (Roto et al., 2015). A stable microflora avoids infections in the gut by preventing colonization using bacterial antagonism, competition by attachment sites or receptors, or interfering with bacterial metabolism. The study of the intestinal microbiota generally includes only the bacteria of the cecum since it has a more favorable environment for microorganisms (lower content of enzymes, antimicrobial compounds, and bile salts); in addition to having an anatomical structure, fermentation, and production of energy metabolites that favor the broiler and its microflora. Fermentation can produce short-chain fatty acids from the starch and fiber of the food, improving digestion and release of energy for the benefit of the body (Roto et al., 2015).
3.9. Effect of yeast on the structure of the digestive system
Several authors reported improved histomorphology, nutrient digestibility, absorption, and other physiological responses when organisms are fed active yeast (Zhang et al., 2005; Gao et al., 2008; Wang et al., 2016a; Bilal et al., 2021; Sun et al., 2021). Bilal et al. (2021) show that incorporating active yeast in diets could promote and protect the structure of the villi, changing the intestinal microbial flora by altering intestinal pH. A stable value of this parameter generates adequate growth of intestinal bacterial communities, helping the digestibility and retention of nutrients that improve health and performance. Changes in the morphology of gastrointestinal microvilli and crypt may indicate the presence of toxins and pathogens that decrease the nutrient absorption surface (small microvilli) and nutritional-energetic wear due to additional cell turnover in gastric tissue. Also, a positive change in microvilli and crypts size increases the area of intentional nutrient absorption, improving growth, weight, FCR, and the antigenic response of the intestinal mucosa (Aluwong et al., 2013; Attia et al., 2020; Sun et al., 2021). Microvilli are projections on the intestinal surface that are covered with mucosa, these increase the absorption of nutrients into the bloodstream through its capillaries. The length and depth of these can change depending on the chicken's diet, stress, and digestive health. Haldar et al. (2011), Attia et al. (2020), Quevedo et al. (2020), He et al. (2021), and Kim et al. (2022) showed that broilers fed a diet supplemented with active Saccharomyces cerevisiae presented a significantly greater amplitude around the crypts of the duodenum and jejunum, a lower number of crypts per millimeter in the duodenum, and higher production of mucus in that tissue compared to the control group. Similar results were obtained by Sun et al. (2021) and Bilal et al. (2021) when using S. boulardii. Deeper crypt increases the regeneration of tissues and microvilli, which increases the absorption of nutrients, increases mucin production reduces the presence of areas affected by pathogens, and reduces the consumption of energy and nutrients used to repair this tissue (Gao et al. 2008; Quevedo et al., 2020). Wang et al. (2017b) evaluated intestinal histomorphology in broiler chickens by supplying active S. cerevisiae, observing an increase in the width of ileal microvilli and their surface area. Wang et al. (2017a) used Kluyveromyces marxianus showing an increase in the ratio between the height of the villi and the depth of the crypts of the jejunum and ileum, in addition to an increase in the height of the ileal villi and sucrase activity, expression of mRNA in the ileum for mucin-2 and sodium-glucose. Similar results are detected in Table 5, where the application of active yeasts positively altered the structure of the microvilli, crypts, and mucin in different parts of the intestine of broiler chickens.
3.10. Effect of yeast on tumor necrosis factor and interleukins
The intestinal surface is a very vulnerable site to pathogens; for this reason, this site has immunolo-gical and non-immunological properties of protection against these pathogens. For example, it has macrophages, dendritic cells, and immune response molecules between and below the surface of the epithelium. Tumor necrosis factor (TNF) is a protein from the group of cytokines released by immune system cells, generating the activation of interleukins (1-6) and is involved in secondary inflammation, apoptosis, and joint destruction. Sun et al. (2017) and Kim et al. (2022) used active S. boulardii and S. cerevisiae, respectively, showing a significant increase in the value of TNF-α and -β present in the duodenum of the organisms treated concerning control (Table 3). Interleukins (IL) are low molecular weight cytokines that function as short-distance chemical messengers in cellular communication. They are generated by leukocytes, endothelial cells, thymus, or bone marrow and function as activators, differentiators, or proliferators of the immune system cells, in addition to the secretion of antibodies, chemotaxis, and regulation of other cytokines. Sun et al. (2017) and Kim et al. (2022) use active yeasts observing an increase in IL levels (10 and 1β, 6, respectively) in the duodenal tissue (Table 3). Interleukins (IL-6), like transforming growth factor (TGF-β), stimulate the production of IgA from B cells of the lamina propria in the intestine, favoring the neutralization of antigens, preventing the binding of pathogens to the intestinal surface and increasing the homeostasis of the intestinal mucosa.
3.11. Yeast effect on digestive enzymes
Broilers have shown different digestive enzymes as carbohydrases (amylases, cellulase, chitinase, maltase), esterases, lipases, and proteases (arylamidase, carboxypeptidase A, B, leucine aminopeptidase, pepsin, trypsin) which are directly associated with feed digestion and nutrient absorption (Aluwong et al., 2013; Ahiwe et al., 2021). Using active yeast for dietary purposes might have stimulated enzyme production in broilers, contributing to development, digestion, nutrition, and health (Bilal et al., 2021). In addition, some yeasts are available to produce extracellular enzymes (amylase, deaminase, dipeptidase, lactate dehydrogenase, lipase, maltase, phospholipase, phosphatase, phytase proteinase, polypeptides, sucrose, transaminase, etc.) and bioactive substances (astaxanthin, -carotenoid, glutathione, polyamine, trehalose, killer toxin, etc.) with potential relevance in digestive processes and nutrient absorption that provide energy to organisms (Magnoli et al., 2016; Ahiwe et al., 2021; Fathima et al. 2023). Sun et al. (2017) used active Saccharomyces boulardii to show that the activity of adenosine triphosphatase, gamma-glutamyl transpeptidase, lipase, and trypsin in the duodenum of the birds was significantly higher compared to the same tissue from the control group. Wang et al. (2017b) fed broilers a diet with active S. cerevisiae and observed a reduction of jejunal maltase activity. Those digestive enzymes play an important role in the catabolism and metabolism of the feed.
3.12. Yeast effect on disease prevention
Diseases caused by pathogens affect the organisms being raised and generate severe economic losses for the broiler industry, which are particularly susceptible to infection by coliforms and other pathogens at the beginning of their lives. The diseases cause weight reduction, food consumption, immunosuppression, and intestinal alteration. An alternative strategy for disease control is the use of active yeasts to improve broiler health and generate resistance to pathogens. Immunosuppression caused by pathogens negatively affects the poultry industry's economy. The use of antibiotics and chemotherapeutics as disease control is undesirable in broiler farms. In addition, vaccines are not effective against many bacterial diseases (Haldar et al., 2011; Ahiwe et al., 2021). Yeast has excellent nutritional content and functional properties, including a role as a probiotic and immune stimulant. Some molecules, such as -glucan and MOS, present in active yeasts have been used to prevent adhesion and colonization of enteric pathogenic bacteria in broilers (Tiago et al., 2012; Wang et al., 2016b; Fathima et al., 2023). Those results have demonstrated the immunostimulatory activity of yeast glucans against broilers viruses, which detected the activation of genes of different immune factors, antimicrobial peptide, anti-lipopolysaccharide factor, and superoxide dismutase (SOD). The production of antioxidant enzymes such as catalase (CAT), glutathione peroxidase (GPx), and SOD in the blood of broiler chickens is needed to remove excess reactive oxygen species (ROS). An increase in the content of antioxidant molecules in broilers' blood can improve the organisms' growth and reduce disease problems (Aluwong et al., 2013; He et al., 2022). In addition, antioxidants prevent the breakdown of serum lipoproteins and contribute to the efficiency of nutrient use during the consumption of these organisms (He et al., 2022). Active yeast cells can stimulate superoxide production by neutrophils and macrophages, increasing the phagocytic capacity of these cells against pathogens. The increase in the expression of antioxidants (superoxide dismutase and glutathione) prevents damage to the structure and function of cell membranes, proteins, and nucleic acids (Wang et al., 2020). Aluwong et al. (2013) reported a significant increase in glutathione peroxidase when using active Saccharomyces cerevisiae in the feed of broiler chickens. He et al. (2021) used S. cerevisiae to detect a significantly high value in the enzyme superoxide dismutase and catalase compared to the control.
Most yeast cell wall proteins are bound to mannan oligosaccharides (MOS), which can act in the gastrointestinal tract of animals as high-affinity binding sites and compete for binding sites against pathogenic bacteria (Mannose-specific type 1 fimbria) (Shashidhara & Devegowda, 2003; Morales-López et al., 2009; Wang et al. 2016b). Avian pathogenic bacteria such as Salmonella sp., Campylobacter sp., and E. coli have strategies of adhesion and colonization of the intestinal mucosa based on type I fimbriae, which are reduced in the presence of active yeasts with MOS (Fathima et al., 2023). These molecules promote the growth of Lactobacillus sp., which neutralizes enterotoxins and inhibits the growth of some pathogens (E. coli, Clostridium sp., Salmonella sp., and Streptococcus sp.) by producing organic acids and reducing intestinal pH resulting in higher nutrient digestion and absorption. He et al. (2021) show that active yeasts can increase the production of a wide range of organic acids in the intestine of broiler chickens, generating an acidic environment that increases digestion and inhibits the growth of pathogenic bacteria. Also, Liu et al. (2022) significantly increased the presence of lactic acid bacteria in the cecum of broiler chickens by adding active D. hansenii to the diets showing that yeast can adhere to the epithelium that reduces the presence of pathogenic bacteria. Haldar et al. (2011) show that the number of Salmonella sp and E. coli decreased in the digest and excreta due to dietary supplementation of S. cerevisiae. They showed a significantly decreased presence of Salmonella sp. and Campylobacter sp. on the neck, breast, cecum, and feces. Wang et al. (2017a) used K. marxianus at different doses showing an abundance reduction of Cyanobacteria, Rickettsiales, Pseudomonadales, and Acinetobacter junii, and an increased abundance of Firmicutes and Lactobacillus sp in the ileum.
4. Current challenges in the use of yeast in broilers chicken
Broiler farming requires new products, techniques, and strategies to increase production and sustainability (FAO, 2023). Yeasts are microorganisms used as nutritional tools in the broiler breeding industry. Depending on the producer's objective, they have different types and applications (Zhang et al., 2005; Gao et al., 2008). However, yeasts function differently in animals depending on the species or strain of yeast used, activity or type of activity it has, whether it is alive or dead, the culture medium where it was produced, whether is it a by-product used, extraction method, and specific application considered (digestion, immunology, nutrition, pathogen control, etc.) (Hana et al., 2015; El-Manawey et al., 2021; Fathima et al., 2023). The species or breed of bird used age, health status, and environment are factors that can also alter the functioning of the yeast. The information from the present study shows that active yeasts play an interesting role in the sustainable cultivation of broilers due to their versatile effects on growth, feed efficiency, intestinal microbiota and structure, and immune response, in addition to increasing the resistance of broilers against diseases (Gao et al., 2008; Kim et al., 2022; Fathima et al., 2023).
Active yeast contains a high level of digestible proteins, amino acids, vitamins (thiamine, riboflavin, nicotinic acid, pantothenic acid, biotin, etc.), minerals (Mg and Zn), and essential elements important for the growth of broilers (Shashidhara & Devegowda, 2003; Gheisari & Kholeghipour 2006; Haldar et al., 2011; Aluwong et al., 2013; Rodríguez et al., 2013; Fanelli et al., 2015; Mousa, 2018; Sun et al., 2021). Chitin, β-glucans, and α-mannans are the main polysaccharides of yeast cell walls, which can interact to promote the elimination of pathogens through microbial antagonism and stimulation of the immune system (Morales-López et al., 2009; Fathima et al., 2023). Different positive effects on poultry production have been associated with ingesting active yeast without fully understanding the exact mechanisms of these effects (Hana et al., 2015). The positive impact of active yeast is the result of an improvement in the functioning of the immune system, use of feed and its respective nutrients, resistance to pathogens, alternative use as growth-promoting antibiotics, and beneficial changes in the intestinal structure of organisms (El-Manawey et al., 2021). Furthermore, the literature shows no consensus on the exact dose or type/mixture of active yeast that should be administered and the feeding duration. Future research should be focused on the exact mechanism of action of these active yeast through which they produce their beneficial effects, as well as their precise dose, type, and duration of feeding.
Broiler farming is an important activity in the food sector, which has rapidly developed and intensified, but its growth has displayed an indiscriminate use of veterinary medicines, antibiotics, and chemical products as prophylactic and control measures for pathogens and diseases (FAO, 2023). This production strategy has resulted in antimicrobial resistance of different pathogens with the risk of spreading. The use of yeast and its products is a possible and viable strategy for preventing and controlling diseases to improve the quality and sustainability of broiler-raising production. Yeast research with specific active products (glucans, MOS, etc.) applies it in low levels (<1% kg broilers diet) and evaluates its effect on target areas of broilers (defense cells, immune system, muscle, etc.) (Shashidhara & Devegowda, 2003; Morales-López et al., 2009; Fathima et al., 2023).
Many studies on active yeast cells and their components have reported improved cellular and humoral responses against pathogens and as a strategy for disease prevention/control (Ahiwe et al., 2021; Kim et al., 2022). Some active yeasts directly stimulate the immune response of broilers, such as phagocytic cells, SOD activity, improvements in antibacterial properties in blood, and mediate signal recognition in cells. Another important element to study is the use of active yeasts to define the specific pathway to improve broiler health by increasing agglutinins, AMP, anti-inflammatory effect, antiapoptotic, bacteriocins, encapsulation, nodule formation and humoral components, polymyxin, siderophores, thyrotricidin, etc. (Pizzolitto et al., 2013).
It is important to increase studies of the effect of some species of active yeast on the structure of the intestine of broiler birds since the microscopic analysis of this tissue shows changes in the structure of the intestinal epithelium (larger size of microvilli and crypts), which improves the absorption of nutrients, in addition to other changes associated with intestinal microflora, anti-inflammatory factors, etc. Also, a long microvillus is correlated with improved gut health. A deeper crypt may signal a more rapid renewal of microvilli affected by inflammation caused by pathogens and their toxins (Adebiyi et al., 2012; Pizzolitto et al., 2013; Wang et al., 2017a). Repairing these damages involves using energy and nutrients that decrease the growth of broiler chickens; for this reason, intestinal health is of great importance in production. The thicker lamina propria and tunica mucosa in the ileum of the control group may be due to inflammation or reactions that occur in the intestine as a defense mechanism against the acquired bacterial load (Robinson et al., 2022). The integrity of the intestinal epithelium and its mucus production is a fundamental barrier to preventing the entry of pathogens. The mucus is composed of mucin (highly glycosylated and interconnected proteins) secreted by goblet cells; it is a barrier where commensal and pathogenic bacteria adhere and colonize. Upon entering the intestine, yeasts colonize the intestinal mucosa by adhering to mucin-binding proteins and, through competitive exclusion, compete with pathogens for the intestinal niche and nutrients (Morales-López et al., 2009).
The gastrointestinal microbiota is important throughout broilers' development, and yeasts may be part of this microbiota (Robinson et al., 2022). The microbiota contributes to the digestion of food and absorption of nutrients throughout the gastrointestinal system, and the different species of yeast have positive effects on the gastrointestinal tract by stimulating the digestive enzymatic process; this constitutes an important element to study in the future. Furthermore, evidence has shown that some yeast species/strains have interesting effects on the gastrointestinal health of broilers, which is related to improved growth, survival, and decreased stress. Future research with new generation technologies (molecular, proteomics, sequencing, real-time PCR) is important to understand the relationship of active yeasts with the microbiota and their ecosystems. It is also important to study the changes presented by the microbiota throughout the growth of broilers, as well as how raising conditions and environmental factors alter it.
Conclusions
The manuscript reveals that Saccharomyces cerevisiae is the main yeast actively used in diets for raising chickens. However, species such as Candida utilis, Debaryomyces hansenii, Kluyveromyces marxianus, Phaffia rhodozyma, and Yarrowia lipolytica are used for the same purpose and generate interesting results. The results show that not all yeast strains function similarly even when they are of the same species and that producers must consider the form of application strain and/or species selected. It is important to establish the specific area that you want to stimulate with yeast to achieve the desired result. Active yeasts are an interesting ingredient that can be used in raising chickens, providing nutritional benefits that improve the growth parameters and intestinal maturity of the birds. They can also enhance the resistance of farmed chickens against diseases by immuno-stimulation the different immune mechanisms of the birds. It is important to consider carrying out more studies on the different species of existing yeasts that can benefit the development of chicken breeding.
Acknowledgments
The authors thank the Universidad Autónoma de Tamaulipas, Facultad de Medicina Veterinaria y Zootecnia for supporting us in this research.
Author Contribution
G. Aguirre-Guzmán: Conceptualization, Research, Analysis, Validation, Writing original-draft, Writing review & editing, Visualization. J. O. Merino-Charrez: Validation, Writing original-draft, Writing review & editing, Visualization. M. L. Torres Rodríguez: Writing original-draft, Writing review & editing, Visualization. M. A. Guevara-Guerrero: Writing original-draft, Writing review & editing, Visualization.
Conflict of Interest Statement
Authors have no conflict of interest to declare.
ORCID
G. Aguirre-Guzmán https://orcid.org/0000-0002-7374-2369
J. O. Merino-Charrez https://orcid.org/0000-0003-1282-8713
M. L. Torres-Rodríguez https://orcid.org/0000-0003-2379-9516
M. A. Guevara-Guerrero https://orcid.org/0009-0000-3651-2516
References
Adebiyi, O. A., Makanjuola, B. A., Bankole, T. O., & Adeyori, A. S. (2012). Yeast culture (Saccharomyces cerevisae) supple-mentation: effect on the performance and gut morphology of broiler birds. Global Journal of Science Frontier Research Biological Sciences, 12(6), 1-6
Ahiwe, E. W., Tedeschi, T. T., Graham, H., & Iji, P. A. (2021). Can probiotic or prebiotic yeast (Saccharomyces cerevisiae) serve as alternatives to in-feed antibiotics for healthy or disease-challenged broiler chickens?: a review Journal of Applied Poultry, 30, 1-13. 100164. https://doi.org/10.1016/j.japr.2021.100164
Alsammar, H., & Delneri, D. (2020). An update on the diversity, ecology, and biogeography of the Saccharomyces genus. FEMS Yeast Research, 20(3), foaa013. https://doi.org/10.1093/femsyr/foaa013
Aluwong, T., Kawu, M., Raji, M., Dzenda, T., Govwang, F., Sinkalu, V., & Ayo, J. (2013). Effect of yeast probiotic on growth, antioxidant enzyme activities and malondialdehyde concentration of broiler chickens. Antioxidants, 2, 326-339. https://doi.org/10.3390/antiox2040326
Attia, Y. A., Al-Khalaifah, H., Abd El-Hamid, H. S., Al-Harthi, M. A., & El-Shafey, A. A. (2020). Growth performance, digestibility, intestinal morphology, carcass traits, and meat quality of broilers fed marginal nutrients deficiency-diet supplemented with different levels of active Yeast. Livestock Science, 233, 103945. https://doi.org/10.1016/j.livsci.2020.103945
Bagust, T. J. (2013). Poultry health and disease control in developing countries. In: Poultry development review. FAO. Rome Italy, 95-98.
Belloch, C., Querol, A., & Barrio, E. (2011). Yeasts and Molds. Kluyveromyces spp. 754-764. In: Encyclopedia of Dairy Sciences. Fuquay J. W. (ed), 2da Edition. Elsevier Ltd. https://doi.org/10.1016/B978-0-12-374407-4.00499-4
Bilal, R. M., Hassan, F. U. I, Saeed, M., Rafeeq, M., Zahra, N., Fraz, A., Saeed, S., Khan, M. A., Mahgoub, H. A. M., Farag, M. R., & Alagawany, M. (2021). Role of yeast and yeast-derived products as feed additives in broiler nutrition. Animal Biotechnology, 34(2), 392-401. https://doi.org/10.1080/10495398.2021.1942028
Cafarchia, C., Latta, R., Danesi, P., Camarda, A., Capelli, G., & Otranto, D. (2018). Yeasts isolated from cloacal swabs, feces, and eggs of laying hens. Medical Mycology, 57, 340–345. https://doi.org/10.1093/mmy/myy026
Cheng, G., Hao, H., Xie, S., Wang, X., Dai, M., Huang, L., & Yuan, Z. (2014). Antibiotic alternatives: the substitution of antibiotics in animal husbandry. Frontiers in Microbiology, 5, 217. https://doi.org/10.3389/fmicb.2014.00217
Churchill, R. R, Mohan, B., & Viswanattran, K. (2000). Effect of supplementation of broiler ratios with live yeast culture. Cheiron, 29, 23-27.
Dedousi, A., Patsios, S. I., Kritsa, M. Z., Kontogiannopoulos, K.N., Ioannidou, M., Zdragas, A., & Sossidou, E. N. (2023). Growth performance, meat quality, welfare, and behavior indicators of broilers fed diets supplemented with Yarrowia lipolytica yeast. Sustainability, 15, 1-24. https://doi.org/10.3390/su15031924
Dixon, B., Kilonzo-Nthenge, A., Nzomo, M., Bhogoju, S., & Nahashon, S. (2022). Evaluation of selected bacteria and yeast for probiotic potential in poultry production. Microorganisms, 10(676), 1-13. https://doi.org/10.3390/ microorganisms10040676
El-Manawey, M. A., Yousif, E. Y., Abo-Taleb, A. M., & Atta, A. M. (2021). The effect of dietary inclusion of whole yeast, extract, and cell wall on production performance and some immunological parameters of broiler chickens. World's Veterinary Journal, 11(2), 257-262. https://dx.doi.org/10.54203/scil.2021.wvj33
Eltazi, S. M., Mohamed, K. A., & Mohamed, M. A. (2014). Response of broiler chicks to diets containing live yeast as probiotic natural feed additive. International Journal of Pharmaceutical Research & Allied Sciences, 3(2), 40-46
Fanelli, A., Agazzi, A., Alborali, G. L., Pilotto, A., Bontempo, V., Dell’Orto, V., Demey, V., Caputo, J. M., & Savoini, G. (2015). Prevalence reduction of pathogens in poultry fed with Saccharomyces cerevisiae. Biotechnology, Agronomy, Society and Environment, 19(1), 3-10.
FAO. (2003). World agriculture: towards 2015/2030, an FAO perspective. Bruinsma J. (Ed). 1er edition. Ed. Routledge. London. pp. 158-176. https://doi.org/10.4324/9781315083858
FAO. (2023). Chickens in 2021. FAOSTAT Statistical Database. Rome. Italy. https://www.fao.org/faostat/en/#data/QCL
Fathima, S., Shanmugasundaram, R., Sifri, M., & Selvaraj, R. (2023). Yeasts and yeast-based products in poultry nutrition. Journal of Applied Poultry Research, 32, 100345. https://doi.org/10.1016/j.japr.2023.100345
Fell, J. W. (2001). Collection and identification of marine yeasts. In: Paul J (ed) Methods in microbiology. Academic Press, New York. pp 347-356. https://doi.org/10.1016/S0580-9517(01)30052-1
Gao, J., Zhang, H. J., Yu, S. H., Wu, S. G., Yoon, I., Quigley, J., Gao, Y. P., & Qi, G. H. (2008). Effects of yeast culture in broiler diets on performance and immunomodulatory functions. Poultry Science, 87, 1377–1384. https://doi.org/10.3382/ps.2007-00418
Gheisari, A., & Kholeghipour, B. (2006). Effect of dietary inclusion of live yeast (Saccharomyces cerevisiae) on growth performance, immune responses, and blood parameters of broiler chickens. Conference paper, 12th European Poultry Conference, Verona Italy.
Grabež, V., Egelandsdal, B., Cruz, A., Hallenstvedt, E., Mydland, L.T., Alvseike, O., Kåsin, K., Ruud, L., Karlsen, V., & Øverland, M. (2022). Understanding metabolic phenomena accompanying high levels of yeast in broiler chicken diets and resulting carcass weight and meat quality changes. Poultry Science, 101, 101749. https://doi.org/10.1016/j.psj.2022.101749
Guardiola, F. A., Esteban, M. A., & Angulo, C. (2021). Yarrowia lipolytica, health benefits for animals. Applied Microbiology and Biotechnology, 105(20), 7577-7592. https://doi.org/10.1007/s00253-021-11584-5
Gul, S. T., & Alsayeqh, A. F. (2023). Probiotics improve physiological parameters and meat production in broiler chicks. International Journal of Veterinary Science, 12(2), 182-191. https://doi.org/10.47278/journal.ijvs/2022.191
Haldar, S., Ghosh, T. K., Toshiwati, & Bedford, M. R. (2011). Effects of yeast (Saccharomyces cerevisiae) and yeast protein concentrate on production performance of broiler chickens exposed to heat stress and challenged with Salmonella enteritidis. Animal Feed Science and Technology, 168, 61–71. https://doi.org/10.1016/j.anifeedsci.2011.03.007
Hana, S. E., Tabidi, M. H., El Nasri, I. M., & Mukhtar, M. A. (2015). Study of different levels of yeast on performance values and immune response in broiler chicken. Journal of Animal Research and Veterinary Science, 8(1), 1-5.
He, T., Mahfuz, S., Piao, X., Wu, D., Wang, W., Yan, H., Ouyang, T., & Li, Y. (2021). Effects of live yeast (Saccharomyces cerevisiae) as a substitute to antibiotic on growth performance, immune function, serum biochemical parameters and intestinal morphology of broilers. Journal of Applied Animal Research, 49(1), 15–22. https://doi.org/10.1080/09712119.2021.1876705
He, T., Ma, J., Mahfuz, S., Zheng, Y., Long, S., Wang, J., Wu, D., & Piao, X. (2022). Dietary live yeast supplementation alleviates transport-stress-impaired meat quality of broilers through maintaining muscle energy metabolism and antioxidant status. Journal of the Science of Food and Agriculture, 102, 4086–4096. https://doi.org/10.1002/jsfa.11758
Hommel, R. K. (2014). Candida introduction. pp 367-373 In: Batt C.A., Tortorello M.L. (ed). Encyclopedia of food microbiology (Second Edition). Academic press. https://doi.org/10.1016/B978-0-12-384730-0.00055-0
Hosseini, S. (2011). The effect of utilization of different levels of Saccharomyces cerevisiae on broiler chicken’s performance. Global Veterinaria, 6(3), 233-236.
Hussein, E., & Selim, S. (2018). Efficacy of yeast and multi-strain probiotic alone or in combination on growth performance, carcass traits, blood biochemical constituents, and meat quality of broiler chickens. Livestock Science, 216, 153–159. https://doi.org/10.1016/j.livsci.2018.08.008
Iraqi, K. G. E., & Fayed, R. H. (2012). Effect of yeast as feed supplement on behavioural and productive performance of broiler chickens. Life Science Journal, 9(4), 4026-4031
Khalifa, W. H., Samy, A., Yassein, S. A., El-Mallah, G., Abusinaa, G. E., & Sallam, M. G. (2024). Using different types of yeast cell extract, probiotic and abiotic to improve growth performance, carcass characteristics and antioxidant activities of broiler chickens. Egyptian Journal of Veterinary Sciences. https://doi.org/10.21608/ejvs.2024.300096.2203
Khan, R. U., & Naz, S. (2013). The applications of probiotics in poultry production. World's Poultry Science Journal, 69, 621-632. https://doi.org/10.1017/S0043933913000627
Kim, E., Kyoung, H., Koh, N. H., Lee, H., Lee, S., Kim, Y., Park, K. II., Heo, J. M., & Song, M. (2022). Supplementation of live yeast culture modulates intestinal health, immune responses, and microbiota diversity in broiler chickens. Journal of Animal Science, 100, 01–11. https://doi.org/10.1093/jas/skac122
Kurtzman, C. P. (2011). Lindnera Kurtzman, Robnett & Basehoar-Powers. (2008). pp 521-543 In: Kurtzman C. P., Fell J. W., Boekhout T. (ed). The yeasts, a taxonomic study (Fifth Edition). Elsevier. https://doi.org/10.1016/B978-0-444-52149-1.00042-2
Laubscher, W. D. F, Laubscher, B. C. Viljoen, & Albertyn, J. (2020). The yeast flora occurring in the trachea of broiler chicken. Food Technology and Biotechnology, 38(1), 77–80
Lawrence-Azua, O. O., Awe, A. O., Saka, A. A., Okotie, U. J., Awodele, O. A., & Isegbe, E. I. (2018). Effect of yeast (Saccharomyces cerevisiae) supplementation on the growth performance, haematological and serum biochemical parameters of broiler chicken. Nigerian Journal of Animal Science, 20(1), 191-199
Liu, C. L., Shih, Y. R., Tang, P. C., Linc, L. J., & Lee, T. T. (2022). Effects of dietary supplementation with Bacillus spp. and Debaryomyces spp. on broiler’s growth performance, serum characteristics, intestinal microflora, and antioxidant activity. Italian Journal of Animal Science, 21(1), 717–728. https://doi.org/10.1080/1828051X.2022.2059022
Nelson, J. R., McIntyre, D. R., Pavlidis, H. O., & Archer, G. S. (2018). Reducing stress susceptibility of broiler chickens by supplementing a yeast fermentation product in the feed or drinking water. Animals, 8, 1-9. https://doi.org/10.3390/ani8100173
Magnoli, A. P., Rodriguez, M. C., Poloni, V. L., Rojo, M. C., Combina, M., Chiacchiera, S. M., Dalcero, A. M., & Cavaglieri, L. R. (2016). Novel yeast isolated from broilers’ feedstuff, gut, and faeces as aflatoxin B1 adsorbents. Journal of Applied Microbiology, 121, 1766-1776. https://doi.org/10.1111/jam.13297
Mirbagheri, M., Nahvi, I., Emtiazi, G., Mafakher, L., & Darvishi, F. (2012). Taxonomic characterization and potential biotechnological applications of Yarrowia lipolytica isolated from meat and meat products. Jundishapur Journal of Microbiology, 5(1), 346-51. https://doi.org/10.5812/kowsar.20083645.2433
Morales-López, R., Auclair, E., García, F., Esteve-Garcia, E., & Brufau, J. (2009). Use of yeast cell walls; β-1, 3/1, 6-glucans; and mannoproteins in broiler chicken diets. Poultry Science, 88, 601–607. https://doi.org/10.3382/ps.2008-00298
Mousa, M. A. M. (2018). Evaluation of using propionic acid and live yeast in diets low in protein and energy on broiler performance. Egyptian Poultry Science, 38, 797-814. https://doi.org/10.21608/EPSJ.2018.17105
Mulatu, K., Ameha, N., & Girma, M. (2019). Effects of feeding different levels of baker’s yeast on performance and hematological parameters in broiler chickens. Journal of World's Poultry Research, 9(2), 38-49. https://dx.doi.org/10.36380/jwpr.2019.5
Osita, C. O., Ani, A. O., Oyeagu, C. E., Akuru, E. A., Ugwuowo, L. C., Udeh, V. C., & Oliobi, U. J. (2020). Effect of different levels of dietary inclusion of Saccharomyces cerevisiae on growth performance and hematological parameters in broiler birds. Bulgarian Journal of Agricultural Science, 26(5), 1024–1028.
Perenlei, G., Tojo, H., Okada, T., Kubota, M., Kadowaki, M., & Fujimura, S. (2014). Effect of dietary astaxanthin rich yeast, Phaffia rhodozyma, on meat quality of broiler chickens. Animal Science Journal, 85, 895–903. https://doi.org/10.1111/asj.12221
Paryad, A., & Mahmoudi, M. (2008). Effect of different levels of supplemental yeast (Saccharomyces cerevisiae) on performance, blood constituents and carcass characteristics of broiler chicks. African Journal of Agricultural Research, 3(12), 835-842.
Patsios, S. A., Dedousi, A., Sossidou, E. N., & Zdragas, A. (2020). Sustainable animal feed protein through the cultivation of Yarrowia lipolytica on agro-industrial wastes and by-products. Review. Sustainability, 12(4), 1398. https://doi.org/10.3390/su12041398
Patterson, R., Rogiewicz, A., Kiarie, E. G., & Slominski, B. A. (2023). Yeast derivatives as a source of bioactive components in animal nutrition: A brief review. Frontiers in Veterinary Science, 9, 1-12. 1067383. https://doi.org/10.3389/fvets.2022.1067383
Pizzolitto, R. P., Armando, M. R., Salvano, M. A., Dalcero, A. M., & Rosa, C. A. (2013). Evaluation of Saccharomyces cerevisiae as an antiaflatoxicogenic agent in broiler feedstuffs. Poultry Science 92. 1655–1663. http://dx.doi.org/10.3382/ps.2012-02846
Quevedo, D. M., Ochoa, J. E., Corredor, J. R., & Pulecio, S. L. (2020). Efectos de la adición de probiótico Saccharomyces cerevisiae sobre histomorfología intestinal en pollos de engorde. Revista de la Facultad de Medicina Veterinaria y de Zootecnia, 67(3), 239-252. https://doi.org/10.15446/rfmvz.v67n3.93931
Rafique, K., Rahman, A., & Mahmood, M. (2020). Effect of dietary supplementation of different levels of Saccharomyces cerevisiae on growth performance and hematology in broiler. Indian Journal of Animal Research, 54(1), 59-64. https://doi.org/10.18805/ijar.B-695.
Rameshwari, K. S., & Karthikeyan, S. (2005). Distillery yeast sludge (DYS) as an alternative feed resource in poultry. International Journal of Poultry Science, 4(10), 787-789. https://doi.org/10.3923/ijps.2005.787.789
Rassmidatta, K., Theapparat, Y., Chanaksorn, N., Carcano, P., Adeyemi, K. D., & Ruangpanit, Y. (2024). Dietary Kluyveromyces marxianus hydrolysate alters humoral immunity, jejunal morphology, cecal microbiota and metabolic pathways in broiler chickens raised under a high stocking density. Poultry Science, 103, 103970. https://doi.org/10.1016/j.psj.2024.103970
Robinson, K., Yang, Q., Stewart, S., Whitmore, M. A., & Zhang, G. (2022). Biogeography, succession, and origin of the chicken intestinal mycobiome. Microbiome, 10, 1–15. https://doi.org/10.1080/00295639.2021.1935103
Rodríguez, B., Valdivié, M., Lezcano, P., & Herrera, M. (2013). Evaluation of torula yeast (Candida utilis) grown on distillery vinasse for broilers. Cuban Journal of Agricultural Science, 47(2), 183-188.
Roto, S. M., Rubinelli, P. M., & Ricke, S. C. (2015). An introduction to the avian gut microbiota and the effects of yeast-based prebiotic-type compounds as potential feed additives. Frontiers in Veterinary Science, 2, 28. https://doi.org/10.3389/fvets.2015.00028
Saied, J. M., Al-Jabary, Q. H., & Thalij K. M. (2011). Effect of dietary supplement yeast culture on production performance and hematological parameters in broiler chicks. International Journal of Poultry Science, 10(5), 376-380. https://doi.org/10.3923/ijps.2011.376.380
Sapsuha, Y., Suprijatna, E., Kismiati, S., & Sugiharto, S. (2021). Combination of probiotic and phythobiotic as an alternative for antibiotic growth promoter for broiler chickens - a review. Livestock Research for Rural Development, 33. http://www.lrrd.org/lrrd33/4/3349yus_ar.html
Sarkar, A., & Bhaskara-Rao, K. V. (2016). Marine yeast: a potential candidate for biotechnological applications- a review. Asian Journal of Microbiology, Biotechnology & Environmental Sciences, 18(3), 6 27-634.
Shankar, P. A., Premavalli, K., Omprakash, A. V., Kirubakaran, J. J., & Hudson, G. H. (2017). Effect of dietary yeast supplementation on the production performance of broilers. International Journal of Applied Business Research, 7(2), 222-228
Shashidhara, R. G., & Devegowda, G. (2003). Effect of dietary mannan oligosaccharide on broiler breeder production traits and immunity. Poultry Science, 82, 1319–1325. https://doi.org/10.1093/ps/82.8.1319
Sun, Y., Rajput, I. R., Arain, M. A., Li, Y., & Baloch, D. M. (2017). Oral administration of Saccharomyces boulardii alters duodenal morphology, enzymatic activity, and cytokine production response in broiler chickens. Animal Science Journal, 88, 1204–1211. https://doi.org/10.1111/asj.12757
Sun, Z., Zhen, Y., Li, T., Aschalew, N. D., Wang, T., Chen, X., Zhao, W., Zhang, X., & Qin, G. (2021). Yeast culture (Saccharomyces cerevisiae) and its active metabolites affect the cecal microbiome of broilers. South African Journal of Animal Science, 51(6), 678-688. https://doi.org/10.4314/sajas.v51i6.1
Sutherland, J. B., Cornelison, C., & Crow, S. A. (2014). Candida, Yarrowia lipolytica (Candida lipolytica). pp 374-378. In: Batt, C. A., Tortorello, M. L. (ed) Encyclopedia of Food Microbiology (Second Edition). Academic Press. https://doi.org/10.1016/B978-0-12-384730-0.00056-2
Tabidi, M. H., Mukhtar, A. M., & Elkhidir, E. E. (2013). Response of chicks for diet containing live yeast as probiotic natural feed additive. Journal of Current Research in Science, 1(5), 316-31. https://doi.org/10.1093/ps/84.7.1015
Tiago, F. C. P., Martins, F. S., Souza, E., Pimenta, P. F. P., Araujo, H. R. C., Castro, I. M., Brandão, R. L., & Nicoli, J. R. (2012). Adhesion to the yeast cell surface as a mechanism for trapping pathogenic bacteria by Saccharomyces probiotics. Journal of Medical Microbiology, 61, 1194–1207. https://doi.org/10.1099/jmm.0.042283-0
Wang, W., Li, Z., Ren, W., Yue, Y., & Guo, Y. (2016a). Effects of live yeast supplementation on lipopolysaccharide-induced inflammatory responses in broilers. Poultry Science, 95, 2557–2564. http://dx.doi.org/10.3382/ps/pew191
Wang, W., Li, Z., Han, Q., Guo, Y., Zhang, B., & D’inca, R. (2016b). Dietary live yeast and mannan-oligosaccharide supplementation attenuate intestinal inflammation and barrier dysfunction induced by Escherichia coli in broilers. British Journal of Nutrition, 116, 1878–1888. https://doi.org/10.1017/S0007114516004116
Wang, W., Li, Z., Lv, Z., Zhang, B., Lv, H., & Guo, Y. (2017a). Effects of Kluyveromyces marxianus supplementation on immune responses, intestinal structure, and microbiota in broiler chickens. PLoS ONE, 12(7), e0180884. https://doi.org/10.1371/ journal.pone.0180884
Wang, W., Ren, W., Li, Z., Yue, Y., & Guo, Y. (2017b). Effects of live yeast on immune responses and intestinal morphological structure in lipopolysaccharide-challenged broilers. Canadian Journal of Animal Science, 97, 136–144. https://doi.org/10.1139/cjas-2015-0148
Wang, D., Wang, D., Pu, L., & Wei, G. (2020). Improved antioxidant capacity and immune function of broiler chickens fed with selenium-enriched Candida utilis. Brazilian Journal of Poultry Science, 22(2), 1-7. https://doi.org/10.1590/1806-9061-2019-1047
Wrent, P., Rivas, E. M., Gil de Prado, E., Peinado, J. M., & de Silóniz, M. I. (2014). Debaryomyces. pp 563-570 In: Batt, C. A., Tortorello M. L. (ed) Encyclopedia of food microbiology (Second Edition). Academic press. https://doi.org/10.1016/B978-0-12-384730-0.00081-1
Zaky, A. S., Tucker, G. A., Daw, Z. Y., & Du, C. (2014). Marine yeast isolation and industrial application. FEMS Yeast Research, 14, 813–825. https://doi.org/10.1111/1567-1364.12158
Zhang, A. W., Lee, B. D., Lee, S. K., Lee, K. W., An, G. H., Song, K. B., & Lee, C. H. (2005). Effects of yeast (Saccharomyces cerevisiae) cell components on growth performance, meat quality, and ileal mucosa development of broiler chicks. Poultry Science, 84, 1015–1021. https://doi.org/10.1093/ps/84.7.1015
Zhang, S., Liao, B., Li, X., Li, L., Ma, L., & Yan, X. (2012). Effects of yeast cell walls on performance and immune responses of cyclosporine A-treated, immunosuppressed broiler chickens. British Journal of Nutrition, 107, 858–866. https://doi.org/10.1017/S000711451100362X