RESEARCH ARTICLE
Sapote gum as a new biopolymer suitable emulsion stabilizer: Grapeseed oil ultrasonic emulsification
Katherin Lloy Arce-Rios1, 2 ; José Luis Pasquel-Reátegui1,** ; Thony Arce-Saavedra3 ;
Eliana Marcela Vélez-Erazo1,*
1 Grupo de Investigación en Ingeniería y Tecnología Agroindustrial, Facultad de Ingeniería Agroindustrial, Universidad Nacional de San Martín (UNSM), Tarapoto, SM, Perú.
2 Department of Food Engineering, School of Food Engineering, University of Campinas, Monteiro Lobato Street, 80, 13083-862, Campinas, SP, Brazil.
3 Departamento Académico de Ciencia y Tecnología Agroindustrial, Facultad de Ciencias Agrarias, Universidad Nacional Autónoma de Chota (UNACH), Chota, Cajamarca, Perú.
* Corresponding author: elianamve@gmail.com (E. M. Vélez-Erazo).
** Corresponding author: jlpasquelr@unsm.edu.pe (J. L. Pasquel-Reátegui).
Received: 19 August 2024. Accepted: 24 December 2024. Published: 14 January 2025.
Abstract
Sapote gum (SG) is a new biopolymer with promissory functional properties. This study aimed to determine if SG is a suitable emulsifier for obtaining stable grape seed oil (GSO) emulsions. In the first stage, coarse emulsion concentrations of SG and grapeseed oil - GSO were evaluated, applying the Central Composite Rotational Design (0.59% to 3.41% of SG and 12.93% to 27.07% GSO). For the second stage, using a Centered Face Design – CFD, the resulting emulsion was sonicated at 90, 270, and 450 Watts at 5, 10, and 15 min. Finally, a validation was made. Emulsions were evaluated through microstructure, droplet size, kinetic stability, heat stress, and rheology. Micrographs of the first-stage emulsions showed droplets up to 3.8 μm diameter and a creaming index between 0.00% and 28.39% after 24 h. Optimization indicates that the higher the concentration of gum (3.5%) and GSO (25%), the more kinetically stable emulsions are produced. Ultrasonic emulsions showed no significant difference in droplet size and kinetic stability before 14 days of rest. Ultrasonic validation was made at 450 W for 6 min, resulting in emulsions stable for 20 days and with rheological characteristics interesting for food or cosmetic industries.
Keywords: Central Composite Rotational Design; Capparis scabrida, Vitis labrusca; stable emulsion; Centred Face Design; ultrasound.
DOI: https://doi.org/10.17268/sci.agropecu.2025.005
Cite this article:
Arce-Rios, K. L, Pasquel-Reátegui, J. L., Arce-Saavedra, T., & Vélez-Erazo, E. M. (2025). Sapote gum as a new biopolymer suitable emulsion stabilizer: Grapeseed oil ultrasonic emulsification. Scientia Agropecuaria, 16(1), 51-59.
1. Introduction
The International Organization of Vine and Wine reported that total wine production in 2023 was 237 million hectoliters (OIV, 2024). In wine production, more than 0.2 kg of pomace is generated for every kilogram of grapes pressed, and the seeds account for 25% of this pomace (Duba & Fiori, 2015). Grapeseed oil (GSO) is a component of pomace from the wine industry. This bioproduct is important to recover because the residue from grape processing is used to do so, which helps conserve the environment and increase the added value to viticulture (Yang et al., 2021).
The GSO contains a significant amount of polyunsaturated fatty acids (PUFAs) ranging from 63.64% - 84.4% in its different grape varieties (Dabetic et al., 2020; Fernandes et al., 2013; Górnaś et al., 2018), mainly linoleic acid; It also contains isomers of vitamin E such as those found in the red Marufo grape variety, α - tocopherol (244 mg/kg of oil), γ - tocopherol (19 mg/kg of oil), α - tocotrienol (319 mg/kg of oil) and γ - tocotrienol (1575 mg/kg of oil) (Fernandes et al., 2013). This composition allows the production of high-quality functional products in order to take advantage of their properties in different presentations such as antimicrobial films (Mauro et al., 2022), biodiesel (Hazar & Sevinc, 2023), emulsions (Mutlu, 2023; Sarabandi et al., 2022), among others.
Food technology requires natural emulsions that come from sustainable and healthy production. Emulsions are generally made using high-energy methods such as ultrasound. Nevertheless, the high-energy emulsification process as a mixing system can cause some undesirable changes in the ingredients of the food emulsion. Thus, it is essential to study the emulsion, emulsifier, and oil phase changes after sonication (Taha et al., 2020; Zhou et al., 2021). Emulsions present significant challenges that must be overcome for their application in industry. Due to emulsions being formed by two immiscible fluids in a non-spontaneous manner, they present thermodynamic instability. In addition, they have physical instabilities such as flocculation, coalescence, Ostwald ripening, phase inversion, and gravitational instabilities, characterized by an irreversible increase in the size of the dispersed phase droplets (Kelmer & Costa, 2024). Emulsifiers and stabilizers, therefore, play an important role in the food industry.
Among the biopolymers studied as GSO emulsifiers are milk protein (Sarabandi et al., 2022; Silva et al., 2020a), gelatin/sodium alginate (Mutlu, 2023), gum arabic (Surini et al., 2018), lupine Protein (Francisco et al., 2023), poppy pollen protein and peptides (Sarabandi et al., 2023), polyglycerol polyglycino-leate polyglycinoleate /whey protein isolate with konjac glucomannan (Zhuang et al., 2023), pectin/ gelatin (Khah et al., 2021). However, no further information exists on the use of sapote gum as a stabilizer or emulsifier. Previous studies have reported that its use as a coating on bananas was promising; it was able to preserve the fruit's characteristics over the storage period (Vélez-Erazo et al., 2022). In addition, as a plasticizer in chitosan films, it has better properties than other natural plasticizers (Gonzaga et al., 2019).
Capparis scabrida is a tree grown in Peru, Ecuador, Venezuela, Colombia, Bolivia, Panama, Brazil, and Costa Rica, from which the gum is extracted from vascular exudations (Herz Castro, 2007). The use of this species is mainly forestry due to the value of its wood, and its deforestation has led the Peruvian government to declare the species in critical danger of extinction and scientists to promote conservation strategies (Abreu-Naranjo et al., 2020; Rodríguez-Rodríguez et al., 2007). The gum has a moisture content of 11% to 12%, water solubility value of 91% to 98%, viscosity of 230 cps at 17%, pH of 4.41 (Herz Castro, 2007), and high in protein (8.45% - 9.3%) and carbohydrates content (83.5% - 86.46%) (Herz Castro, 2007; Moscol Ortiz, 2018). Thus, its application as a stabilizer represents an interesting alternative due to its biodegradable presentation compared to synthetic polymers and toxic preservatives.
For all the above, the objective of this research was to study, for the first time, the sapote gum potential to form and stabilize grapeseed oil emulsion using ultrasound as an emulsion-forming technique.
2. Methodology
Material
Grape seed oil (Vitis labrusca) was purchased by the company Nature, located in the province of San Martín, department of San Martín, Peru, which uses burgundy grape seeds from the viticulture of local producers. The oil was kept refrigerated until use. The sapote gum (Capparis scabrida) (protein (8.45% - 9.3%) and carbohydrate content (83.5% - 86.46%) (Herz Castro, 2007; Moscol Ortiz, 2018)) was provided by local producers in the Almirante Miguel Grau, located in Piura, Peru.
Obtention of pre-emulsion
The sapote gum and water solution were left in magnetic stirring for 24 h, then GSO was added. An Ultraturrax rotor-stator (Ika, Werke GmbH and Co. KG, Staufenim Breisgau, Germany) was used to form the emulsion by stirring at 12000 rpm for 2 minutes, and the emulsion was immediately analyzed by optical microscopy and kinetic stability for 24 hours. The different proportions of sapote gum and GSO were combined according to the Central Composite Rotational Design (CCRD) type design between 0.59% and 3.41%, and 12.93% and 27.07%, respectively (Table 1).
Obtention of ultrasonic-emulsion
The optimal emulsion of the previous assay was subjected to ultrasound (900W full power, 20 kHz frequency, Branson 250, USA, probe (Ø = 12.5 mm)) at different conditions of power amplitude (10%, 30% and 50%), which provided nominal powers of 90, 270, and 450 Watts, respectively; and time (5, 10, and 15 min) according to the Centred Face Design (CFD) generating 12 treatments (Table 2). The ultrasonic emulsion was immediately analyzed by optical microscopy and kinetic stability for 0, 7, and 14 days (the emulsion was kept under laboratory conditions until the analysis was performed).
Characterization of pre-emulsion and ultrasonic emulsion
Kinetic stability
For the analysis of kinetic stability, we proceeded according to Vélez-Erazo et al. (2018), where 40 mL of each previous emulsion was placed in 100 mL graduated tubes and then left in ambient conditions for 24 hours. Phase formation was then observed, and the oil phase volume was recorded every 30 minutes for the first 3 hours and at 24 hours. The details of the kinetic stability analysis are shown in Figure 1.
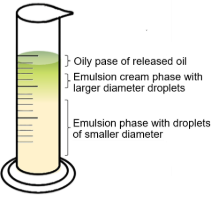
Figure 1. Phases observed in kinetic stability analysis.
The kinetic stability was calculated according to the oil release (OR), creaming index (CI), and Creaming - Oil index (COI) given in equations 1, 2, and 3) which is defined between the ratio of the height of the oil phase, creaming phase or creaming-oil phase of the emulsion (H) and the initial height (Ho).
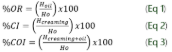
Where Ho represents the initial volume of the emulsion, and H is the volume of the upper or cream phase. The CI was determined in duplicate, and the results were expressed as mean ± standard deviation.
For the analysis of the kinetic stability of the ultrasonic emulsion, 25 mL of each treatment was placed in graduated specimens of 50 mL, where the emulsions were analyzed on days 0, 7, and 14; in addition, for this last day, the ultrasonic emulsions were subjected to thermal stress in a water bath at 90 °C for 20 minutes, following the methodology of Li et al. (2023).
Optical microscopy and droplet diameter
It was determined by optical microscopy using a microscope (Nikon, Eclipse Ni-U model) with an amplitude of 40x (previous emulsions) and 100x with immersion oil (ultrasonic emulsions), with image capture through the digital camera controlled by the Motic Images Plus program. For the analysis of the micrographs, the ImageJ software was used, which allowed for determining the area of the droplets (400 drops/treatment) and, from this, calculated the theoretical average diameter according to equation 4 according to Saout et al. (1999).
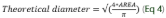
Table 1
Central Composite otational Design (CCRD) and characteristics of pre-emulsions
Exp | Gum (%) | Oil (%) | Oil release | Creaming index | Creaming - Oil index | Diameter (µm) |
1 | -1(1) | -1(15) | 7.14±0.91 | 9.74±0.92 | 16.88±0.00 | 4.94±3.10 |
2 | 1(3) | -1(15) | 0.00±0.00 | 8.11±0.00 | 8.11±0.00 | 3.79±2.75 |
3 | -1(1) | 1(25) | 6.58±1.86 | 14.47±1.86 | 21.05±0.00 | 4.72±4.07 |
4 | 1(3) | 1(25) | 0.00±0.00 | 0.00±0.00 | 0.00±0.00 | 4.51±2.09 |
5 | -1.41(0.59) | 0(20) | 15.07±0.00 | 9.59±0.00 | 24.66±0.00 | 4.90±2.44 |
6 | 1.41(3.41) | 0(20) | 0.00±0.00 | 1.35±0.00 | 2.01±0.93 | 3.98±1.87 |
7 | 0(2) | -1.41(12.93) | 2.67±0.05 | 10.67±0.20 | 13.34±0.25 | 4.11±2.00 |
8 | 0(2) | 1.41(27.07) | 2.58±0.02 | 28.39±0.26 | 30.97±0.28 | 4.87±3.03 |
9 | 0(2) | 0(20) | 3.21±0.97 | 13.46±0.66 | 16.67±0.30 | 5.33±3.53 |
10 | 0(2) | 0(20) | 3.21±0.97 | 13.46±0.66 | 16.67±0.30 | 4.97±3.06 |
11 | 0(2) | 0(20) | 3.20±0.85 | 12.82±0.23 | 16.02±0.61 | 5.00±3.18 |
12 | 0(2) | 0(20) | 2.58±0.02 | 14.19±0.13 | 16.77±0.15 | 4.24±2.31 |
Table 2
Central Face Design (CFD) and Particle Diameter (μm)
T | X1 (Watts) | X2 (Min) | D0 | D7 | D14 | WB |
1 | -1 (90) | -1 (5) | 1.60±0.73 | 2.37±1.18 | 2.05±0.81 | 1.80±0.87 |
2 | 1 (450) | -1(5) | 1.90±0.69 | 1.86±1.07 | 1.55±0.61 | 1.61±0.39 |
3 | -1(90) | 1(15) | 2.10±0.92 | 1.75±0.90 | 1.49±0.51 | 1.41±0.35 |
4 | 1(450) | 1(15) | 2.09±0.49 | 1.73±0.64 | 1.42±0.60 | 1.85±0.54 |
5 | -1(90) | 0(10) | 1.82±0.76 | 1.30±0.47 | 1.54±0.56 | 1.36±035 |
6 | 1(450) | 0(10) | 1.84±0.59 | 1.64±0.62 | 1.36±0.47 | 2.00±0.93 |
7 | 0(270) | -1(5) | 1.81±0.71 | 1.64±0.60 | 1.69±0.50 | 1.59±0.37 |
8 | 0(270) | 1(15) | 1.65±0.71 | 1.82±0.74 | 1.25±0.32 | 1.70±0.40 |
9 | 0(270) | 0(10) | 1.42±0.58 | 1.92±0.82 | 1.73±0.44 | 1.63±0.50 |
10 | 0(270) | 0(10) | 2.04±0.78 | 1.59±0.52 | 1.65±0.48 | 1.79±0.56 |
11 | 0(270) | 0(10) | 1.61±0.711 | 1.24±0.45 | 1.58±0.43 | 1.53±0.34 |
12 | 0(270) | 0(10) | 1.77±0.59 | 1.56±0.82 | 1.49±0.35 | 1.82±0.70 |
D0: particle diameter day 0, D7: particle diameter day 7, D14: particle diameter day 14, and WB: particle diameter after water bath (heat stress).
Similar results were obtained by Sepeidnameh et al. (2024). The authors analyzed the stability of GSO-multilayer emulsions stabilized by gelatin, gelatin-chitosan, and gelatin-chitosan-basil seed gum homogenized only by a rotor-stator device. All emulsions presented phase separation at day 7, reaching values of 36%, 46%, and 53% of the creaming index for emulsions stabilized by gelatin, gelatin-chitosan, and gelatin-chitosan-basil seed gum, respectively.
Characterization of ultrasonic emulsion
The purpose of using a Centred Face Design (CFD) was to establish the optimal ultrasonic power and time application conditions for the formation of stable emulsions using optimized pre-emulsion of sapote gum (3.50%) and GSO (25%). These concentrations were defined by analyzing Figure 3, with the aim to obtain less oil release (Figure 3A), less cream formation (Figure 3B and C), and the smallest droplet size (Figure 3D). These responses were analyzed to determine the greatest possible GSO incorporation. In response to this step, the ultrasound-emulsion characterization is presented in Figure 4 and Table 2.
As shown in Figure 4, the ultrasonic emulsions presented similar microstructure and monomodal droplet size distribution. Also, these emulsions did not exhibit creaming phase formation, indicating that the emulsions had good kinetic stability during the evaluation period (14 days), even after heat stress. Results coincided with the study of Silva et al. (2015) on ultrasound's effectiveness in producing kinetically stable single or double emulsions (Li et al., 2023).
In some treatments, the development of dark-colored colonies was observed on day 7, possibly due to the presence of microorganisms, which could cause the collapse of interfacial protein structure, the oxidation of lipids, and finally, the destabilization of the emulsion (Yuan et al., 2013). However, in this case, their presence on the emulsions until day 14 did not signify an instability factor. Given the good stability of this emulsion, it is recommended that antimicrobials be used for future work.
In relation to particle size, it is observed that on day 0, the diameter varies between 1.418μm and 2.092 μm, on day 7 between 1.239 μm and 2.369 μm, on day 14 between 1.251 μm and 2.054 μm, and after heat stress between 1.359 μm and 1.998 μm (Table 2).
Statistical results reveal that on days 0 and 7, there were no significant effects of variables (power and sonication time) on droplet size due to the low R2 observed (Table 4). The droplet size was so homogeneous that no difference was observed when applying a statistical analysis, which does not necessarily mean something negative. In contrast, after 14 days, the sonication time variable appears to be a slightly influential factor in droplet size and a higher degree of correlation with droplet diameter. This behavior implies that the longer the emulsion rest time, the more the droplet structure is reshaped. Similar studies suggest optimal conditions for producing 10-day stable sonoemulsions at GSO (10% w/w) and protein solutions (3.3% w/w) with casein:whey protein ratios of 60:40, 50:50, 40:60 produced with an energy density of 81.9 J mL−1 (500 W at 60% amplitude for 7 minutes) (Silva et al., 2019, 2020a).
Table 4
Reparametrized model for ultrasound-emulsion’ droplet size
Diameter | Polynomial Equations | R2 |
Day 0 | Y1= 1.70 +0.06 x1 + 0.16 x12 + 0.09 x2 +0.05 x22 – 0.07 xxx2 | 38.30 |
Day 7 | Y2 = 1.53 – 0.03 x1 + 0.05x12 – 0.09 x2 + 0.30 x22 +0.12 x1 x2 | 45.09 |
Day 14 | Y3= 1.56 – 0.13 x1 – 0.18 x2 + 0.11 x1 x2 | 73.38 |
The results of the droplet diameter of the emulsion after 14 days of resting show that there is a significant difference between the treatments and, in the contour graph (Figure 4C), the tendency is observed that the longer the time and power of sonication, the smaller the particles are formed. These results express a polynomial equation based on a correlation greater than 70%. Before 14 days, it was not possible to define a mathematical model that would respond to the optimization of the process because the warhead did not compromise the two variables.
Silva et al. (2020b) obtained GSO-ultrasound emulsions stabilized with milk proteins. The authors found that ultrasound can form small droplets (<3 μm), which is related to high emulsion stability. These results are similar to those found in the present study, highlighting the potential of sapote gum to form and stabilize GSO emulsions since sapote gum presented an emulsifying behavior similar to an excellent emulsifier such as milk proteins.
Validation
A final validation experiment was made after variable analyses. As all emulsions were stable for 14 days (without phase separation), ultrasound parameters were determined at 450 W for 6 min, calculating a droplet size < 1.5 μm (Figure 4C). At these conditions, low process time and high potency avoid overprocessing (increasing the temperature) and allow work at low energy density, reducing the energy cost (Vélez-Erazo et al., 2018). Results show that the final assay is stable for 20 days without phase separation or another destabilization process (Figure 5A).
Figure 5B-F shows the rheological characterization. Several models are widely used for fitting experimental flow data, such as Ostwald, Casson, Mizarhi-Berk, and Herschel Buckley (Gamboa-Alarcón et al., 2023). Experimental data presented a good adjustment to the Herschel-Bulkley model (R2>0.99) and showed a shear-thinning behavior due to the flow behavior index (n) being a little higher than 1 (1.19). Additionally, apparent viscosity decreases as the shear rate increases.
On the other hand, in oscillatory assays, loss modulus (G'') was higher than storage modulus (G'), indicating that emulsion was predominantly viscous material affected mainly by frequency. Figure 5E demonstrates that SG-emulsion is also characterized as a concentrated solution without gel behavior despite showing a clear tendency for elastic-like behavior at higher frequencies (Steffe, 1996).
Finally, the thixotropy test evaluates the deformation and regenerative capacity of the emulsion, presenting a recovery percentage of 73% (Calculated as the relation of the final viscosity of the first interval and the first viscosity of the third interval). An acceptable recovery percentage (over 70%) was obtained, suggesting that this emulsion can be used in systems requiring reversible deformation (Wu et al., 2022).
4. Conclusions
Sapote gum (Capparis scabrida) is a new biopolymer that demonstrated emulsifying capacity, maintaining grapeseed oil-emulsion stability during the evaluated time (20 days). Two experimental designs were established using response surface methodology to study the variables’ modeling and the optimal values for formulation (rotor-stator pre-emulsions) and ultrasonic parameters (Ultrasonic emulsions).
In the rotor-stator pre-emulsions, some experiments presented phase separation after a few hours of storage, and droplet size varied between 3.79 and 5.33 μm. The optimized pre-emulsion was defined as 3.5% sapote gum and 25% grapeseed oil. Ultrasonic emulsions did not present phase separation during 14 days of storage, even after heat treatment. Ultrasound decreases the droplet size (1.25 – 2.37 μm), ensuring emulsion stability.
Finally, an emulsion’ validation was made with 3.5% sapote gum and 25% grapeseed oil and with ultrasonic parameters of 450 W for 6 min. This emulsion resulted in a shear-thinning fluid with predominantly viscous behavior, being a concentrated solution without gel behavior and with reversible deformation. All these characteristics allow the incorporation of sapote gum-stabilized emulsion in a wide range of products.
Future investigations are necessary because there are no studies on the application of sapote gum as a biopolymer in different industries, such as food, cosmetics, or pharmaceuticals.
Author's contribution
K. L. Arce-Rios: Conceptualization, Methodology, Formal Analysis, Visualization, Writing - Review & Editing Original Draft. J. L. Pasquel Reátegui: Supervision, Conceptualization, Project administration, Funding acquisition, Writing – Review & Editing. T. Arce-Saavedra: Writing - Review & Editing Original Draft. E. M. Vélez-Erazo: Supervision, Conceptua-lization, Writing - Review & Editing.
Funding Declaration
This study was financially supported by Instituto de Investigación (IDI-UNSM-PERÚ) Resolución N° 623-2022-UNSM/CU-R.
Data Availability
The authors confirm that the data supporting the findings of this study are available within the article.
Declarations of Conflict of Interest
The authors declare no competing interests.
ORCID
K. L. Arce-Rios https://orcid.org/0000-0003-1933-9533
J. L. Pasquel-Reátegui https://orcid.org/0000-0001-6467-394X
T. Arce-Saavedra https://orcid.org/0000-0002-2300-9169
E. M. Vélez-Erazo https://orcid.org/0000-0002-8632-5329
References
Abreu-Naranjo, R., Ramirez-Huila, W. N., Reyes Mera, J. J., Banguera, D. V., & León-Camacho, M. (2020). Physico-chemical characterisation of Capparis scabrida seed oil and pulp, a potential source of eicosapentaenoic acid. Food Bioscience, 36, 100624. https://doi.org/10.1016/J.FBIO.2020.100624
Dabetic, N. M., Todorovic, V. M., Djuricic, I. D., Stankovic, J. A. A., Basic, Z. N., Vujovic, D. S., & Sobajic, S. S. (2020). Grape Seed Oil Characterization: A Novel Approach for Oil Quality Assessment. European Journal of Lipid Science and Technology, 1900447, 1–10. https://doi.org/10.1002/ejlt.201900447
Duba, K. S., & Fiori, L. (2015). Supercritical CO2 extraction of grape seed oil: Effect of process parameters on the extraction kinetics. The Journal of Supercritical Fluids, 98, 33–43. https://doi.org/10.1016/J.SUPFLU.2014.12.021
Fernandes, L., Casal, S., Cruz, R., Pereira, J. A., & Ramalhosa, E. (2013). Seed oils of ten traditional Portuguese grape varieties with interesting chemical and antioxidant properties. Food Research International, 50(1), 161–166. https://doi.org/10.1016/j.foodres.2012.09.039
Francisco, C. R. L., Santos, T. P., & Cunha, R. L. (2023). Nano and micro lupin protein-grape seed extract conjugates stabilizing oil-in-water emulsions. Food Hydrocolloids, 135, 108117. https://doi.org/10.1016/J.FOODHYD.2022.108117
Gamboa-Alarcón, P. W., Enriquez-Castillo, D. F., Suyón-Martínez, J. A., & Rodríguez-Paúcar, G. N. (2023). Comportamiento reológico de la pulpa de mango (Mangifera indica L.) liofilizada con encapsulantes. Revista Agrotecnológica Amazónica, 3(1), e436. https://doi.org/10.51252/raa.v3i1.436
Gonzaga, A., Rimaycuna, J., Cruz, G. J. F., Bravo, N., Gómez, M. M., Solis, J. L., & Santiago, J. (2019). Influence of natural plasti-cizers derived from forestry biomass on shrimp husk chitosan films. Journal of Physics: Conference Series, 1173(1). https://doi.org/10.1088/1742-6596/1173/1/012006
Górnaś, P., Rudzińska, M., Grygier, A., & Lācis, G. (2018). Diversity of oil yield, fatty acids, tocopherols, tocotrienols, and sterols in the seeds of 19 interspecific grapes crosses. Journal of the Science of Food and Agriculture, 99(5), 2078–2087. https://doi.org/10.1002/JSFA.9400
Hazar, H., & Sevinc, H. (2023). Investigation of exhaust emissions and performance of a diesel engine fueled with preheated raw grape seed oil/propanol blends. Chemical Engineering and Processing - Process Intensification, 188, 109378. https://doi.org/10.1016/J.CEP.2023.109378
Herz Castro, K. B. (2007). Análisis físico-químico de la goma exudada de la especie sapote (Capparis scabrida H.B.K.), proveniente de los bosques secos de Lambayeque. In Universidad Nacional Agraria La Molina. http://repositorio.lamolina.edu.pe/handle/20.500.12996/403
Kelmer, F., & Costa, F. F. (2024). Innovations and stability challenges in food emulsions. Sustainable Food Technology. https://doi.org/10.1039/D4FB00201F
Khah, M. D., Ghanbarzadeh, B., Roufegarinejad Nezhad, L., & Ostadrahimi, A. (2021). Effects of virgin olive oil and grape seed oil on physicochemical and antimicrobial properties of pectin-gelatin blend emulsified films. International Journal of Biological Macromolecules, 171, 262–274. https://doi.org/10.1016/J.IJBIOMAC.2021.01.020
Li, J., Wang, S., Wang, H., Cao, W., Lin, H., et al. (2023). Effect of ultrasonic power on the stability of low-molecular-weight oyster peptides functional-nutrition W1/O/W2 double emulsion. Ultrasonics Sonochemistry, 92, 106282. https://doi.org/10.1016/j.ultsonch.2022.106282
Mauro, M., Pinto, P., Settanni, L., Puccio, V., Vazzana, M., Hornsby, B. L., et al. (2022). Chitosan Film Functionalized with Grape Seed Oil—Preliminary Evaluation of Antimicrobial Activity. Sustainability, 14(9), 5410. https://doi.org/10.3390/SU14095410
Medeiros, A. M., Vélez-Erazo, E. M., Grossi, G., Furtado, G. D. F., Vidotto, D. C., Tavares, G. M., & Hubinger, M. D. (2022). High internal phase emulsions stabilized by the lentil protein isolate (Lens culinaris). Colloids and Surfaces A : Physicochemical and Engineering Aspects, 653(June), 129993. https://doi.org/10.1016/j.colsurfa.2022.129993
Moscol Ortiz, J. A. (2018). Caractetización física-química para determinación del rendimiento y calidad de la goma exudada de la especie forestal sapote Capparis scabrida H.B.K en el área de conservación regional angostura faical. Universidad Nacional de Tumbes.
Mutlu, N. (2023). Effects of grape seed oil nanoemulsion on physicochemical and antibacterial properties of gelatin-sodium alginate film blends. International Journal of Biological Macromolecules, 237, 124207. https://doi.org/10.1016/J.IJBIOMAC.2023.124207
OIV. (2024). State of the world vine and wine sector in 2023.
Rodríguez-Rodríguez, E. F., Bussmann, R. W., Jackeline, S., Alfaro, A., & López, E. (2007). Capparis scabrida (Capparaceae) una especie del Perú y Ecuador que necesita planes de conservación urgente Capparis scabrida (Capparaceae) a species from Peru and Ecuador in urgent. https://www.researchgate.net/publication/228108008
Saout, C., Quéré, C., Donval, A., Paulet, Y. M., & Samain, J. F. (1999). An experimental study of the combined effects of temperature and photoperiod on reproductive physiology of Pecten maximus from the Bay of Brest (France). Aquaculture, 172(3–4), 301–314. https://doi.org/10.1016/S0044-8486(98)00406-2
Sarabandi, K., Akbarbaglu, Z., Mazloomi, N., Gharehbeglou, P., Peighambardoust, S. H., & Jafari, S. M. (2023). Structural modification of poppy-pollen protein as a natural antioxidant, emulsifier and carrier in spray-drying of O/W-emulsion: Physicochemical and oxidative stabilization. International Journal of Biological Macromolecules, 250, 126260. https://doi.org/10.1016/j.ijbiomac.2023.126260
Sarabandi, K., Tamjidi, F., Akbarbaglu, Z., Samborska, K., Gharehbeglou, P., Kharazmi, M. S., & Jafari, S. M. (2022). Modification of Whey Proteins by Sonication and Hydrolysis for the Emulsification and Spray Drying Encapsulation of Grape Seed Oil. Pharmaceutics, 14(11), 2434. https://doi.org/10.3390/PHARMACEUTICS14112434/S1
Sepeidnameh, M., Fazlara, A., Hosseini, S. M. H., et al. (2024) Encapsulation of grape seed oil in oil-in-water emulsion using multilayer technology: Investigation of physical stability, physicochemical and oxidative properties of emulsions under the influence of the number of layers. Current Research in Food Science 8(February). 100771. https://doi.org/10.1016/j.crfs.2024.100771
Silva, E. K., Azevedo, V. M., Cunha, R. L., Hubinger, M. D., & Meireles, M. A. A. (2015). Ultrasound-assisted encapsulation of annatto seed oil: Whey protein isolate versus modified starch. Food Hydrocolloids, 56, 71–83. https://doi.org/10.1016/j.foodhyd.2015.12.006
Silva, M., Zisu, B., & Chandrapala, J. (2019). Interfacial and emulsification properties of sono-emulsified grape seed oil emulsions stabilized with milk proteins. Food Chemistry, 309, 125758. https://doi.org/10.1016/j.foodchem.2019.125758
Silva, M., Zisu, B., & Chandrapala, J. (2020a). Stability of oil–water primary emulsions stabilised with varying levels of casein and whey proteins affected by high-intensity ultrasound. International Journal of Food Science and Technology, 56(2), 897–908. https://doi.org/10.1111/ijfs.14737
Silva, M., Zisu, B., & Chandrapala, J. (2020b) Interfacial and emulsification properties of sono-emulsified grape seed oil emulsions stabilized with milk proteins. Food Chemistry, 309(2019). 125758. https://doi.org/10.1016/j.foodchem.2019.125758
Steffe, J. F. (1996). Rheological Methods in food process engineering (F. Press, Ed.; 2nd ed.). Freeman Press. https://doi.org/10.1016/0260-8774(94)90090-6
Surini, S., Azzahrah, F. U., & Ramadon, D. (2018). Microencapsulation of grape seed oil (Vitis vinifera L.) with gum arabic as a coating polymer by crosslinking emulsification method. International Journal of Applied Pharmaceutics, 10(6), 194–198. https://doi.org/10.22159/IJAP.2018V10I6.24093
Taha, A., Ahmed, E., Ismaiel, A., Ashokkumar, M., Xu, X., Pan, S., & Hu, H. (2020). Ultrasonic emulsification: An overview on the preparation of different emulsifiers-stabilized emulsions. Trends in Food Science and Technology, 105, 363–377. https://doi.org/10.1016/j.tifs.2020.09.024
Vélez-Erazo, E. M., Bosqui, K., Rabelo, R. S., Kurozawa, L. E., & Hubinger, M. D. (2020). High internal phase emulsions (HIPE) using pea protein and different polysaccharides as stabilizers. Food Hydrocolloids, 105. https://doi.org/10.1016/j.foodhyd.2020.105775
Vélez-Erazo, E. M., Carbajal-Sandoval, M. S., Sanchez-Pizarro, A. L., Peña, F., Martínez, P., & Velezmoro, C. (2022). Peruvian Biopolymers (Sapote Gum, Tunta, and Potato Starches) as Suitable Coating Material to Extend the Shelf Life of Bananas. Food and Bioprocess Technology, 15(11), 2562–2572. https://doi.org/10.1007/s11947-022-02902-4
Vélez-Erazo, E. M., Consoli, L., & Hubinger, M. D. (2018). Mono and double-layer emulsions of chia oil produced with ultrasound mediation. Food and Bioproducts Processing, 112, 108–118. https://doi.org/10.1016/j.fbp.2018.09.007
Wu, J., Guan, X., Wang, C., Ngai, T., & Lin, W. (2022). pH-Responsive Pickering high internal phase emulsions stabilized by Waterborne polyurethane. Journal of Colloid and Interface Science, 610, 994–1004. https://doi.org/10.1016/J.JCIS.2021.11.156
Yang, C., Shang, K., Lin, C., Wang, C., Shi, X., Wang, H., & Li, H. (2021). Processing technologies, phytochemical constituents, and biological activities of grape seed oil (GSO): A review. Trends in Food Science & Technology, 116, 1074–1083. https://doi.org/10.1016/J.TIFS.2021.09.011
Yuan, Y., Wan, Z., Yin, S., & Yang, X. (2013). Stability and antimicrobial property of soy protein/chitosan mixed emulsion at acidic condition. Food & Function, 4(9), 1394. https://doi.org/10.1039/c3fo60139k
Zhou, L., Zhang, J., Xing, L., & Zhang, W. (2021). Applications and effects of ultrasound assisted emulsification in the production of food emulsions: A review. Trends in Food Science and Technology, 110(1), 493–512. https://doi.org/10.1016/j.tifs.2021.02.008
Zhuang, H., Li, X., Wu, S., Wang, B., & Yan, H. (2023). Fabrication of grape seed proanthocyanidin-loaded W/O/W emulsion gels stabilized by polyglycerol polyricinoleate and whey protein isolate with konjac glucomannan: Structure, stability, and in vitro digestion. Food Chemistry, 418, 135975. https://doi.org/10.1016/j.foodchem.2023.135975