The soil used in the study was classified as Fluvic Neosol (EMBRAPA, 2013), and samples were taken to the Soil, Water and Limestone Analysis Laboratory - LASAC, where its chemical characterization was carried out, whose results are shown in Table 1.
Before differentiating the soil water availability (SWA) levels, inoculated and non-inoculated seeds were placed to germinate in the soil, and the pots were irrigated so as to maintain SWA at field capacity (SWA level of 100% FC) until obtaining a seedling with the first true leaf, a period that extended to 17 days after sowing (DAS). From the appearance of the true leaf until the end of the watermelon cycle (72 DAS), the volume of water applied began to be differentiated to maintain the SWA of these treatments.
Irrigation management was carried out using a TDR100 model from Campbell (Time Domain Reflectometry) equipment. Four different probes were selected, one for each type of inoculation. After reading, the treatments were manually irrigated using a graduated cylinder. In the period of SWA differentiation, irrigation was based on replacing the volume of water consumed to the levels evaluated in the study, aiming to maintain the limits established in the treatments.
Two strains of the genus Bacillus spp., named XX6.9 and P6.2, from the collection of the Microbial Biotechnology Laboratory, were used. In previous studies conducted by the Laboratory’s research group, the strains have shown efficiency in environments with water restrictions for the maize crop, and plant growth-promoting potential in monocots. Both bacterial strains were previously isolated from the rhizosphere of cacti native to the Caatinga (Dias et al., 2022; Silva et al., 2023).
The bacterial isolates were cultured for 48h at 28 ± 2 °C. For the microbiolization of the seed, xanthan gum solution was added to the cultures, after the concentrations had been individually adjusted by spectrophotometry model UV-1600 from Pró-Análise (OD 540 nm). Subsequently, the seeds of watermelon var. Crimson Sweet were immersed in the suspension for 40 min.
The seeds used as control were immersed in xanthan gum solution to be subjected to the same procedure as the other treatments. After this period, the seeds were left to dry on paper towels at room temperature.
Three seeds per pot were sown at 3 cm depth, in order to obtain one plant per pot after thinning.
The biochemical variables total soluble sugars (TSS) (Yemm & Willis, 1954), reducing sugars (RS) (Miller, 1959), total proteins (Pt) (Bradford, 1976) and proline (Pr) (Bates et al., 1973), as well as the enzymatic variables catalase (CAT) (Peixoto, 1999) and peroxidase (POD) (Teisseire & Guy, 2000), and the oxidative stress marker, lipid peroxidation (MDA) (Heath & Packer, 1968), were measured at fruiting (72 DAS).
To determine the enzymatic activity, the plant material was collected, wrapped in aluminum foil, properly identified, and frozen in liquid nitrogen (±196 °C) to immediately paralyze all metabolic reactions. Subsequently, the samples were stored in a freezer for further analysis of enzyme activity.
The data were subjected to analysis of variance (ANOVA) with F test at 5% and 1% significance levels. When significant, the means were compared by regression analysis (soil water availability) and Scott-Knott test (inoculation of bacteria), using SISVAR software version 4.0 (Ferreira, 2011).
3. Results and discussion
In the analysis of variance, soil water availability (SWA) levels caused significant effects on all enzymatic and biochemical variables. For the bacterial inoculation, all enzymatic and biochemical variables were influenced, except for reducing sugars (RS). In addition, significant interaction was observed between SWA and bacterial inoculations for antioxidant enzymes and biochemical factors.
Regarding lipid peroxidation (MDA) (Figure 2) as a function of SWA, the regression curves of the evaluated treatments followed a quadratic behavior, except for the treatment P6.2, which showed a linear and decreasing response.
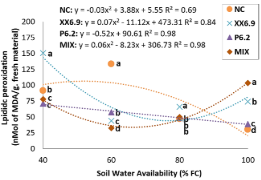
Figure 2. Lipid peroxidation of watermelon inoculated (XX6.9, P6.2 and MIX) or not (NC) subjected to soil water availability levels, at 72 DAS. Means followed by identical letters do not differ from each other by the Scott-Knott test at 5% significance level. Juazeiro, BA, 2024.
The highest lipid peroxidation in Negative Control (NC) (106.56 nMol MDA g fresh matter-1) occurred with SWA level of 52% FC, with a subsequent reduction with increasing SWA, reaching 30.24 nMol MDA g fresh matter-1, with 100% field capacity (FC) (Figure 2).
The content of MDA, the end product of lipid peroxidation, is used to determine the extent of oxidative damage. If the MDA content increased, it means that reactive oxygen species (ROS) were generated, leading to membrane disturbance, which can cause oxidative stress to proteins, nucleic acids, lipids, and the electron transport chain (Ashagari et al., 2020; Alkahtani et al., 2021a). When analyzing the MDA content between the treatments as a function of SWA alone (Figure 2), it was found that the lowest value at SWA of 40% FC was obtained with the P6.2 treatment, while the lowest value at SWA of 60% FC was obtained with the MIX treatment, decreasing almost 22 and 76%, respectively, compared to NC.
This is not the first time that inoculation in Cucurbitaceae species has contributed to reduction of MDA under water stress. Alkahtani et al. (2021a) explain that the better performance with inoculation is due to the increase in phenols and greater enzymatic activity, which protect proteins and lipids from oxidative stress, neutralizing ROS, reducing the formation of MDA, and preventing the cell membrane from being damaged.
On the other hand, the XX6.9 and NC treatments had higher MDA content than the other treatments at the respective SWA levels (40 and 60% FC) (Figure 2). Abbasi et al. (2020) also observed the same behavior with bacterial treatments. However, the parameters of vegetative growth were not affected by water stress. Therefore, it is important to evaluate other parameters to determine the efficacy of the inoculation.
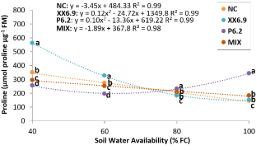
Figure 3. Proline in the leaf tissue of watermelon inoculated (XX6.9, P6.2 and MIX) or not (NC) subjected to soil water availability levels, at 72 DAS. FC – Field capacity. Means followed by identical letters do not differ from each other by the Scott-Knott test at 5% significance level. Juazeiro, BA, 2024.
Figure 3 shows the behavior of proline as a function of SWA. For the treatments with XX6.9 bacteria, MIX and NC, the regression showed an increase in proline as SWA decreased (Figure 3). In addition, the increase in proline was more pronounced with the XX6.9 treatment (Figure 3), whose mean was 563.67 μmol proline μg-1 FM at the SWA level of 40% FC, with an increase of 61% compared to NC. Lu et al. (2021) demonstrated that the mean of the XX6.9 treatment was even higher than that observed in the watermelon crop without inoculation at -50 kPa, for which the means ranged from 150 to 200 μmol proline μg-1 FM. The increase in proline constitutes an instantaneous response to water stress, as this organic acid acts as an antioxidant and a signaling molecule, preventing the action of ROS. It acts as an osmotic regulator, reducing the osmotic potential of the cell and, in the case of root cells, allows water uptake even in harsh environments (Cui et al., 2021).
Plants are already equipped with an antioxidant defense that includes SOD (superoxide dismutase) and APX (ascorbate peroxidase), in addition to CAT and POD. However, under severe and prolonged conditions, the system is not sufficient to prevent damage. Therefore, inoculation with Bacillus would have a positive effect on inducing enzymes, alleviating oxidative damage (Anee et al., 2019; Latef et al., 2020), as observed in the present study.
When analyzing peroxidase (POD) as a function of SWA, it was observed that the treatments were influenced by the SWA levels, and the regression showed a linear trend, except for the P6.2 treatment, whose response was quadratic (Figure 4).
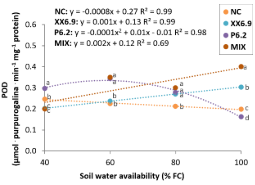
Figure 4. Peroxidase (POD) of watermelon inoculated (XX6.9, P6.2 and MIX) or not (MIX) subjected to soil water availability levels, at 72 DAS. Means followed by identical letters do not differ from each other by the Scott-Knott test at 5% significance level. Juazeiro, BA, 2024.
The P6.2 treatment proved to be superior in water-restricted environments compared to the other treatments, with maximum POD (0.34 μmol purpurogallin min-1 mg-1 protein) at the SWA level of 59.5% (Figure 4). This result was lower than that obtained in cantaloupe melon (Cucumis melo L.) inoculated with a mix (Pseudomonas, Azotobacter and Enterobacter cloacae), which averaged 1.1 μmol purpurogallin min-1 mg-1 protein at 50% FC (Zahedyan et al., 2022).
In relation to the test of means for POD, when analyzing the applied treatments at each SWA level separately (Figure 4), it was observed that the P6.2 treatment showed a higher POD activity at the SWA level of 40% FC. On the other hand, at the SWA levels of 60 and 80% FC, the P6.2 and MIX treatments did not show significant difference, but POD activity was higher at both SWA levels. This is compatible with what was expected for the treatments, since both reduced MDA contents at the aforementioned SWA levels (P6.2 and MIX at SWA levels of 40 and 60% FC, respectively) (Figure 2).
The increase in enzyme activity when accompanied by a reduction in MDA content leads to a decrease in oxidative stress under water-stressed conditions (La et al., 2019). Bikdeloo et al. (2021) state that low MDA contents in ‘Crimson Sweet’ watermelon and the greater induction of the enzyme peroxidase are indicative of its better ability to cope with oxidative stress.
When analyzing the influence of SWA as a function of the treatments with bacteria on catalase activity, the treatments NC, P6.2 and MIX showed a quadratic behavior (Figure 5).
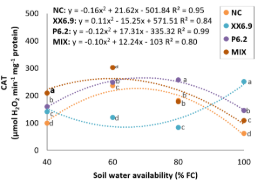
Figure 5. Catalase (CAT) of watermelon inoculated (XX6.9, P6.2 and MIX) or not (NC) subjected to soil water availability levels, at 72 DAS. Means followed by identical letters do not differ from each other by the Scott-Knott test at 5% significance level. Juazeiro, BA, 2024.
NC, P6.2 and MIX treatments showed maximum CAT activity (226.24; 264.99; 262.41 μmol H2O2 min-1 mg-1 protein, respectively) at the respective SWA levels of 67, 69 and 60% FC (Figure 5). It was observed that the MIX treatment induced higher activity compared to NC at a lower SWA. When analyzing the means of CAT in the applied treatments as a function of SWA alone (Figure 5), it can be seen that the MIX increased CAT by 22% compared to NC at the SWA level of 60% FC.
When employing a bacterial mix, one must consider the metabolism of each strain and that inoculation affects the growth of the coexisting strains, leading to interactions, which can be neutral, positive (cooperation, synergism, commensalism) or negative (amensalism, competition, antagonism). Therefore, compatibility between strains it is fundamental (Moreno-Galván et al., 2020; Thomloudi et al., 2019), which explains the good performance of MIX in relation to CAT.
Another characteristic to consider is the sampling origin of the strains. Ideally, the site should come from native plants that grew in arid environments and co-evolved with their microbiota, contributing for the strains to be adapted, tolerant, with long survival and persistence in diverse environments (Gamalero & Glick, 2022), a condition consistent with the materials used in this study.
In addition to the strain-to-strain interaction, another factor is the compatibility between the strains and the watermelon crop. Stress minimization depends on a specific plant-strain interaction, and this influence becomes evident through enzymatic activity and lipid peroxidation (Azeem et al., 2022; Pinski et al., 2019).
The means of CAT in the applied treatments as a function of SWA alone (Figure 5) show that CAT activity with the MIX treatment was also higher at the SWA level of 40% FC (208.92 μmol H2O2 min-1 mg-1 protein). This result is associated with the fact that CAT is located in all ROS-producing compartments and works as a fine regulator converting H2O2 into H2O+½O2. Therefore, the higher the CAT activity, the more efficient the system and the greater its capacity to decompose H2O2 (Jayaraj & Beevy, 2021; Sood et al., 2020).
About total protein (Figure 6), a decreasing linear regression can be observed for the NC and XX6.9 treatments, i.e., they increase protein content with the reduction of SWA.
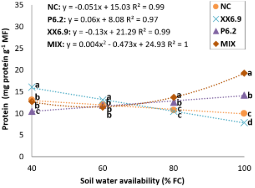
Figure 6. Total proteins of the watermelon crop inoculated (XX6.9, P6.2 and MIX) or not (NC) subjected to soil water availability levels, at 72 DAS. Means followed by identical letters do not differ from each other by the Scott-Knott test at 5% significance level. Juazeiro, BA, 2024.
NC obtained an average protein content of 13.04 mg g-1 with SWA of 40% FC (Figure 6), consistent with that obtained in yellow melon cv. Gold Mine, which showed increase in proteins under 50% FC (16.94 mg g-1), with a subsequent decrease under 100% FC, revealing a constitutive characteristic of the variety, which may justify, in part, a drought tolerance strategy (Cruz et al., 2022). XX6.9 bacteria also increased protein content at SWA levels from 100 to 40% FC, from 7.88 to 16.01 mg g-1, respectively (Figure 6), and led to a protein production higher than that obtained with NC at the SWA range from 40 to 60% FC.
Protein synthesis is induced by bacteria in order to adjust the osmotic potential, ensure the normal functioning of plant physiological processes, and inhibit protein degradation (Mendoza-Labrador et al., 2021; Prgomet et al., 2020; Sheteiwy et al., 2021). Thus, it is also interesting to note that the XX6.9 treatment increased protein content between SWA levels of 40 and 60% FC, as well as proline content (Figure 6). This is an indication that the treatment with XX6.9 has a greater capacity to synthesize these solutes under water restriction.
Proteins and proline are osmotic regulators, which maintain water content and protect cells, and because proline is made up of hydrophilic and hydrophobic sections, it can affect protein solubility and prevent albumin abnormality. Studies have already shown the relationship between proline and the surface of proteins, increasing stability and preventing modifications in their structure (Alkahtani et al., 2021b). Thus, as the XX6.9 treatment had a higher proline content, this may also have been a determining factor for protein maintenance.
For total soluble sugars as a function of SWA levels, regression analysis showed that the mathematical model that best fitted to the XX6.9 treatment was the decreasing linear model (Figure 7).
The XX6.9 treatment induced a higher production of TSS at SWA levels with water restriction (Figure 7). Regarding the interference of treatments in TSS as a function of the SWA analyzed individually (Figure 7), it can be seen that the XX6.9 treatment increased TSS by 149; 304 and 91% compared to NC at SWA levels of 40; 60 and 80% FC, respectively.
TSS accumulation is a strategy to withstand damage to cell organelles, proteins, and membranes and increase the stability of cell membranes to provide a stable environment for photosynthetic reaction, act on osmotic balance, and allow plants to absorb water from the soil (Lu et al., 2020; Vieira et al., 2020).
Although the P6.2 and MIX treatments did not increase TSS values above the XX6.9 treatment, they were superior to NC at the SWA range from 60 to 80% FC (Figure 7). This response demonstrates that inoculation, regardless of inoculant, increases TSS in water-limiting environments.
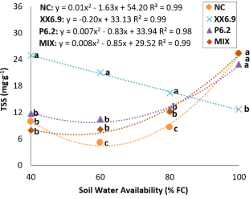
Figure 7. Total soluble sugars (TSS) of watermelon inoculated (XX6.9, P6.2 and MIX) or not (NC) subjected to soil water availability levels, at 72 DAS. Means followed by identical letters do not differ from each other by the Scott-Knott test at 5% significance level. Juazeiro, BA, 2024.
In this context, it should be noted that one of the impacts of water stress is the impairment of physiological activities, such as water absorption, cell division, CO2 assimilation, and reduction in the supply of assimilates, including sucrose (Alkahtani et al., 2021a). However, this was not the case for plants inoculated with the bacteria.
When analyzing reducing sugars (RS) as a function of SWA levels, it was observed that the quadratic model was the one that best fitted to the data (Figure 8).

Figure 8. Reducing sugars (RS) of watermelon inoculated (XX6.9, P6.2 and MIX) or not (NC), subjected to soil water availability levels, at 72 DAS. Means followed by identical letters do not differ from each other by the Scott-Knott test at 5% significance level. Juazeiro, BA, 2024.
As observed in the RS content (Figure 8), the XX6.9 treatment also showed higher RS at the SWA level of 60% FC, more limiting than the other treatments. The highest RS content (3.08 mg g-1 FM) was obtained at 68% of the soil water retention capacity. Vieira et al. (2020) also observed quadratic behavior in melon cv. ‘Piel de sapo’ under 50; 75; 100 and 125% ETc, with maximum RS (6.22 mg g-1 FM) at 50% ETc.
Table 2 shows the difference between TSS and RS, determined by the decrease in the TSS variable by RS in inoculated and non-inoculated treatments.
Table 2
Difference between total soluble sugars (TSS) and reducing sugars (RS) in watermelon crop
| TSS - RS |
| 40% | 60% | 80% | 100% |
NC | 7.86 | 3.62 | 6.36 | 21.23 |
XX6.9 | 22.61 | 18.16 | 13.70 | 10.99 |
P6.2 | 9.36 | 9.74 | 10.87 | 17.22 |
MIX | 5.69 | 6.97 | 10.21 | 20.72 |
The XX6.9 treatment was responsible for the highest synthesis of TSS under SWA levels of 40; 60 and 80% FC, showing its capacity to synthesize total soluble sugars (Table 2).
In summary, the P6.2 and MIX treatments induced the activation of enzymes under low water availability, while the XX6.9 treatment was responsible for the synthesis of osmoprotectants (proline, TSS, RS and total proteins). As observed for XX6.9, the treatments with Bacillus endophyticus, Bacillus altitudinis and Bacillus megaterium also synthesized more osmolytes instead of activating antioxidant enzymes (Devarajan et al., 2021).
When plants reach a severe stress level, the accumulation of solutes has a high energy cost, and the solutes tend to be degraded, leading the plant to use other mechanisms to survive (Santos et al., 2020), such as the enzyme system, so the XX6.9 treatment may not have been efficient in this study.
In addition, it was observed that bacterial treatments induced more POD than CAT, because this enzyme represents a better defense. CAT plays an overall role in tolerance by minimizing H2O2 accumulation, while POD, in addition to draining H2O2, participates in lignin synthesis. Lignin is responsible for increased structural rigidity, durability of tissues, water transport in xylem vessels, physical barrier against pathogens, and complexation of carbohydrates and proteins (Bezerra et al., 2020).
4. Conclusions
The results show that the XX6.9 bacteria and NC treatments were the most affected by severe water stress, since at the SWA level of 40% FC they showed high contents of the oxidative marker (MDA) and proline. Although inoculation with XX6.9 bacteria promoted a higher content of osmoregulators such as proteins, TSS and RS, this treatment was not sufficient to attenuate the effects of water deficit. On the other hand, the treatments with P6.2 bacteria and MIX of bacteria showed reduced levels of MDA at the soil water availability level of 40% FC, accompanied by the high enzymatic activity of POD and CAT, contributing to the tolerance of the watermelon crop to water stress.
Future studies could further investigate the long-term impact of these treatments under field conditions and explore their effectiveness in different soil types and climatic regions. Furthermore, unraveling the molecular mechanisms underlying the interaction between these bacteria and the plant's response to stress can provide deeper knowledge for the development of effective agricultural practices.
Acknowledgments
This study was financed in part by the Coordination for the Improvement of Higher Level Personnel (CAPES) – Finance Code 001.
Authors’ Contributions
Conceptualization: Simões W. L., Mesquita A. C. Data curation: Simões W. L., Mesquita A. C., Araújo, M. G. Formal analysis: Simões W. L., Mesquita A. C., Araújo M. G. Funding acquisition: Simões W. L., Mesquita A. C. Methodology: Simões W. L., Mesquita A. C., Araújo M. G., Carvalho R. N., Felix A. T. R., Silva J. S. Project administration: Simões W. L., Mesquita A. C. Supervision: Mesquita A. C. Writing-original draft: Simões W. L., Mesquita A. C., Araújo M. G. Writing-review & editing: Simões W. L., Mesquita A. C., Araújo M. G., Carvalho R. N., Felix A. T. R., Silva J. S.
Policy on Conflict of Interest
There are no potential personal, commercial, political, academic or financial conflicts of interest. There is no use of artificial intelligence.
ORCID
M. G. de Araújo https://orcid.org/0000-0002-2144-2196
A. C. Mesquita https://orcid.org/0000-0002-9754-1676
W. L. Simões https://orcid.org/0000-0003-1474-9410
R. N. de Carvalho https://orcid.org/0000-0002-8751-6215
A. T. R. Felix https://orcid.org/0000-0003-1071-1490
J. S. da Silva https://orcid.org/0000-0003-3409-0326
References
Abbasi, S., Sadeghi, A., & Safaie, N. (2020). Streptomyces alleviate drought stress in tomato plants and modulate the expression of transcription factors ERF1 and WRKY70 genes. Scientia Horticulturae, 265(109206), 1-9. https://doi.org/10.1016/j.scienta.2020.109206
Alkahtani, M. D., Hafez, Y. M., Attia, K., Rashwan E, Husnain L. A., Algwaiz, H. I., & Abdelaal, K. A. (2021b). Evaluation of silicon and proline application on the oxidative machinery in drought-stressed sugar beet. Antioxidants, 10(398), 1-19. https://doi.org/10.3390/antiox10030398
Alkahtani, M., Hafez, Y., Attia, K., Al-Aateeq, T., Ali, M. A., Hasanuzzaman, M., & Abdelaal, K. (2021). Bacillus thuringiensis and silicon modulate antioxidant metabolism and improve the physiological traits to confer salt tolerance in lettuce. Plants, 10(1025), 1-16. https://doi.org/10.3390/plantas10051025
Anee, T.I., Nahar, K., Rahman, A., Mahmud, J. A., Bhuiyan, T. F., Alam, M. U., Fujita, M., & Hasanuzzaman, M. (2019). Oxidative damage and antioxidant defense in Sesamum indicum after different waterlogging durations. Plants, 8(7), 196-214. https://doi.org/10.3390/plantas8070196
Azeem, M., Haider, M. Z., Javed, S., Salim, M. H., & Alatawi, A. (2022). Drought stress amelioration in maize (Zea mays L.) by inoculation of Bacillus spp. strains under sterile soil conditions. Agriculture, 12(1), 50-71. https://doi.org/10.3390/agriculture12010050
Bates, L. S., Waldren, R. A., & Teare, I. D. (1973). Rapid determination of free proline for water-stress studies. Plant and soil, 39, 205-207. https://doi.org/10.1007/BF00018060
Bezerra, J. D. C., França, S. A., Nascimento Júnior, J. R.S. do., Castro, F. M. de., Silva, N. V. da, & Barbosa, S. N. (2020). Biossíntese de lignina em plantas submetidas ao déficit hídrico. Pubvet, 14(9), 1-14. https://doi.org/10.31533/pubvet.v14n9a653.1-14
Bhadrecha, P., Singh, S., Dwibedi, V. (2023). ‘A plant’s major strength in rhizosphere’, the plant growth promoting rhizobacteria. 2023. Archives of Microbiology, 205(5), 165-190. https://doi.org/10.1007/s00203-023-03502-2
Bikdeloo, M., Colla, G., Rouphael, Y., Hassandokht, M. R., Soltani, F., Salehi, R., Kumar, P., & Cardarelli, M. (2021). Morphological and physio-biochemical responses of watermelon grafted onto rootstocks of wild watermelon [Citrullus colocynthis (L.) Schrad] and commercial interspecific cucurbita hybrid to drought stress. Horticulturae, 7(10), 359-371. https://doi.org/10.3390/horticulturae7100359
Bradford, M. M., (1976). A rapid and sensitive method for quantification of microgran quantities of protein utilizing the principle of protein-dye-binding. Anal. Biochem, 72, 248-254. https://doi.org/10.1006/abio.1976.9999
Cui, G., Xiao, X., Zhang, W., Lang, D., Li, Z., & Zhang, X. (2021). Exogenous silicon relieve drought stress and salt stress of Glycyrrhiza uralensis seedlings by regulating proline metabolism and nitrogen assimilation. The Journal of Horticultural Science and Biotechnology, 96(6), 728-737. https://doi.org/10.1080/14620316.2021.1921624
Cruz, C., Cardoso, P., Santos, J., Matos, D., & Figueira, E. (2022). Bioprospecting Soil Bacteria from Arid Zones with Plant to Increase Plant Tolerance to Drought, Growth and Biochemical Status of Maize Inoculated Growth-Promoting Bacteria Isolated from Sal Island, Cape Verde. Plants, 11(21), 2912-2931. https://doi.org/10.3390/plants11212912
Dias, K., C., F., P., Souza, I., J., S., Dinas, S. S. E., Oliveira, M. C. B., Ferreira, V. Q., Barros, Y. C., & Jesus Santos, A. F de. (2022). Proteção para a cultura de milho contra a seca mediada por bactérias da Caatinga. Agrometeoros, 30, 1-7. https://doi.org/10.31062/agrom.v30.e026986
Devarajan, A. K., Muthukrishanan, G., Truu, J., Truu, M., Ostonen, I., Kizhaeral, S. S., Panneerselvam, P., & Gopalasubramanian, S. K. (2021). The foliar application of rice phyllosphere bacteria induces drought-stress tolerance in Oryza sativa (L.). Plants, 10(2), 387-408. https://doi.org/10.3390/plants10020387
Dubey, A., Kumar, A., Malla, M. A., Chowdhary, K., Singh, G., Ravikanth, H. G., Harish Sharma, S., Saati-Santamaria, Z., Menéndez, E., & Dames, J. F. (2021). Approaches for the amelioration of adverse effects of drought stress on crop plants. Frontiers in Bioscience-Landmark, 26(10), 928-947. https://doi.org/10.52586/4998
EMBRAPA - Empresa Brasileira de Pesquisa Agropecuária. Sistema brasileiro de classificação de solos. 3ª edição. Brasília, DF, 2013. 353 p.
Ferreira, D. F. (2011). Sisvar: A computer statistical analysis system. Ciência e Agrotecnologia, 35(6), 1039-1042. https://doi.org/10.1590/S1413-70542011000600001
Gamalero, E., & Glick, B. R. (2022). Recent advances in bacterial amelioration of plant drought and salt stress. Biology, 11(3), 437-463. https://doi.org/10.3390/biologia11030437
Heath, R. L., & Packer. (1968). Photoperoidation in isolated chloroplasts I. Kinetic and stoichiometry of fatt acid peroxidation. Arch. Biochem. Biophys, 125, 189-198. https://doi.org/10.1016/0003-9861(68)90654-1
Jayaraj, M. S., & Beevy, S. S. (2021). Impact of drought on the characteristics attributes in the varieties of Momordica Charantia L. International Journal of Botany Studies, 6(3), 125-131.
Kamer, M. E. A., Khalil, G. A., & Yousry, M. M. (2022). Yield and Quality Performance of Some New Sweet Melon Lines Under Water Stress Conditions. Journal of the Advances in Agricultural Researches, 27(1), 148-161. https://doi.org/10.21608/jalexu.2022.114554.1036
La, V. H., Lee, B. R., Zhang, Q., Park, S. H., Islam, M. T., & Kim, T. H. (2019). Salicylic acid improves drought-stress tolerance by regulating the redox status and proline metabolism in Brassica rapa. Horticulture, Environment, and Biotechnology, 60(1), 31-40. https://doi.org/10.1007/s13580-018-0099-7
Latef, A. A. H. A., Zaid, A., Abo-baker, A. B. A. E., Salem, W., & Abu Alhmad, M. F. (2020). Mitigation of copper stress in maize by inoculation with Paenibacillus polymyxa and Bacillus circulans. Plants, 9(11), 1513-1531. https://doi.org/10.3390/plantas9111513
Lu, K., Sun, J., Li, Q., Li, X., & Jin, S. (2021). Effect of cold stress on growth, physiological characteristics, and calvin-cycle-related gene expression of grafted watermelon seedlings of different gourd rootstocks. Horticulturae, 7(10), 391-404. https://doi.org/10.3390/horticulturae7100391
Lu, J., Nawaz, M. A., Wei N., Cheng, F., & Bie, Z. (2020). Suboptimal temperature acclimation enhances chilling tolerance by improving photosynthetic adaptability and osmoregulation ability in watermelon. Horticultural Plant Journal, 6(1), 49-60. https://doi.org/10.1016/j.hpj.2020.01.001
Martínez-Valderrama, J., Olcina, J., Delacámara, G., Guirado, E., & Maestre, F. T. (2023). Complex Policy mixes are needed to cope with Agricultural Water demands under Climate Change. Water demands under Climate Change. Water Resources Management, 37(6), 2805-2834. https://doi.org/10.1007/s11269-023-03481-5
Maslennikova, D., & Lastochkina, O. (2021). Contribution of ascorbate and glutathione in endobacteria Bacillus subtilis-mediated drought tolerance in two Triticum aestivum L. genotypes contrasting in drought sensitivity. Plants, 10(12), 2557-2569. https://doi.org/10.3390/plantas10122557
Mendoza-Labrador, J., Romero-Perdomo, F., Abril, J., Hernández, J. P., Uribe-Vélez, D., & Buitrago, R. B. (2021). Bacillus strains immobilized in alginate macrobeads enhance drought stress adaptation of guinea grass. Rhizosphere, 19(14), 1-9 https://doi.org/10.1016/j.rhisph.2021.100385
Miller, G. L. (1959). Use dinitrosalicylis acid reagent for determination of reducing sugars. Analytical Chemistry, 31(2), 426-428. https://doi.org/10.1021/ac60147a030
Mohan, A., Shanmugam, S., & Nithyalakshmi, V. (2024). Comparison of the nutritional, physico-chemical and anti-nutrient properties of freeze and hot air dried watermelon (Citrullus lanatus) Rind. Biosciences Biotechnology Research Asia, 13(2), 1113-1119. http://dx.doi.org/10.13005/bbra/2140
Moreno-Galván, A. E., Cortés-Patiño, S., Romero-Perdomo, F., Uribe-Vélez, D., Bashan, Y., & Bonilla R. R. (2020). Proline accumulation and glutathione reductase activity induced by drought-tolerant rhizobacteria as potential mechanisms to alleviate drought stress in Guinea grass. Applied Soil Ecology, 147, 1-9. https://doi.org/10.1016/j.apsoil.2019.103367
Olotu, Y., Okodugha, D. A., Olarinde, O., Momoh, V. E., & Ibrahim, R. (2024). Estimation of consumptive water use for Watermelon in Auchi, Nigeria. Studia Universitatis Babes-Bolyai Engineering, 69(1). https://doi.org/10.24193/subbeng.2024.1.20
Peixoto, H. P. P., Cambraia, J., Sant’ana, R., Mosquim, P. R., & Moreira, A. M. (1999). Aluminium effects on lipid peroxidation and the activities of enzymes of oxidative metabolism in sorghum. Revista Brasileira de Fisiologia Vegetal, 11(3), 137-143.
Pinski, A., Betekhtin, A., Hupert-Kocurek, K., Mur, L. A., & Hasterok, R. (2019). Defining the genetic basis of plant–endophytic bacteria interactions. International Journal of Molecular Sciences, 20(8), 1947-1979. https://doi.org/10.3390/ijms20081947
Prgomet, I., Pascual-Seva, N., Morais, M. C., Aires, A., Barreales, D., Ribeiro, A. C., Silva, A. P., Barrosa, A. I. R. N. A, & Gonçalves, B. (2020). Physiological and biochemical performance of almond trees under deficit irrigation. Scientia Horticulturae, 261, 1-11. https://doi.org/10.1016/j.scienta.2019.108990
Santos Junior, J. L. dos, Oliveira, M. F. da C., & Silva, E. C. da. (2020). Acúmulo de solutos orgânicos em mudas de Ceiba glaziovii (Kutze) Kum. em resposta à seca intermitente. Scientia Plena, 16(1). https://doi.org/10.14808/sci.plena.2020.011201
Sheteiwy, M. S., Abd Elgawad, H., Xiong, Y. C., Macovei, A., Brestic, M., Skalicky, M., Hiba, Y. A., & El‐Sawah, A. M. (2021). Inoculation with Bacillus amyloliquefaciens and mycorrhiza confers tolerance to drought stress and improve seed yield and quality of soybean plant. Physiologia Plantarum, 172(4), 2153-2169. https://doi.org/10.1111/pp.13454
Silva, D. M. R., Barros, A. C., Silva, R. B., Galdino, W. D. O., Souza, J. W. G. D., Marques, I. C. D. S., ... & Rodrigues, J. D. (2024). Impact of Photosynthetic Efficiency on Watermelon Cultivation in the Face of Drought. Agronomy, 14(5), 950. https://doi.org/10.3390/agronomy14050950
Silva, E. P da., Barros, Y. C., & Santos, A. F. de J. (2023). Mitigação do déficit hídrico em plantas de milho por Bacillus sp. isolados de plantas endêmicas da caatinga. Scientific Electronic Archives, 16(6), 1-7. https://doi.org/10.36560/16620231508
Sood, G., Kaushal, R., & Sharma, M. (2020). Alleviation of drought stress in maize (Zea mays L.) by using endogenous endophyte Bacillus subtilis in North West Himalayas. Acta Agriculturae Scandinavica, Section B—Soil & Plant Science, 70(5), 361-370. https://doi.org/10.1080/09064710.2020.1743749
Teisseire, V. H., & Guy, V. Y. (2000). Copper-induced changes in antioxidant enzymes activities in fronds of duckweed (Lemna minor). Plant Science, 153, 65–72. https://doi.org/10.1016/S0168-9452(99)00257-5
Thomloudi, E. E., Tsalgatidou, P. C., Douka, D., Spantidos, T. N., Dimou, M., Venieraki, A., & Katinakis, P. (2019). Multistrain versus single-strain plant growth promoting microbial inoculants-The compatibility issue. Hell. Plant Prot. J., 12(6), 61–77. https://doi.org/10.3390/biology12060779
Vieira, M. L., Cunha, A. J. da., & Souza, D. S. (2021). Organomineral associado a Bacillus aryabhattai como atenuador do déficit hídrico em mudas de café. Revista Vitae-Educação, Saúde e Meio Ambiente, 1(9): 319–328. https://doi.org/10.17648/2525-2771-v1n9-9
Vieira, D. A., Carvalho, M. M. P., Rodrigues, B. A., Marinho, L. B., & Mesquita, A. C. (2020). Metabolic behavior in the allocation of biomass of melon cultivars under water deficit conditions. Research, Society and Development, 9(8), 1-18. https://doi.org/10.33448/rsd-v9i8.5128
Yadav, V. K., Yadav, R. C., Choudhary, P., Sharma, S. K., & Bhagat, N. (2022). Mitigation of drought stress in wheat (Triticum aestivum L.) by inoculation of drought tolerant Bacillus paramycoides DT-85 and Bacillus paranthracis DT-97. Journal of Applied Biology and Biotechnology, 10(1), 59-69. https://doi.org/10.7324/JABB.2022.10s109
Yemm, E. W., & Willis, A. J. (1954). The estimation of carbohydrates in plants extracts by antrone. Biochemical Journal, 57(3), 508-514. https://doi.org/10.1042/bj0570508
Zahedyan, A., Jahromi, A. A., Zakerin, A., Abdossi, V., & Torkashvand, A. M. (2022). Nitroxin bio-fertilizer improves growth parameters, physiological and biochemical attributes of cantaloupe (Cucumis melo L.) under water stress conditions. Journal of the Saudi Society of Agricultural Sciences, 21(1), 8-20. https://doi.org/10.1016/j.jssas.2021.06.017