1. Introduction
Wetlands associated with tropical river habitats sustain diverse specialized flora and fauna (Chakraborty et al., 2023), acting as essential longitudinal and transversal corridors. These ecosystems facilitate biodiversity dispersion and provide critical ecological functions, including water retention, flood regulation, purification processes, and resources for fishing and forestry (Ghosh et al., 2024). Coastal wetlands encompass a variety of environments, both natural and artificial, that are characterized by being permanently or temporarily inundated by fresh, estuarine (brackish) or saline water, and include marine regions that do not exceed 6 meters in depth with respect to sea level. middle of the sea, as established in the 1971 RAMSAR Convention.
In Ecuador, the "Abras de Mantequilla" wetland has been designated as a Ramsar site since March 14, 2000, which implies that the biodiversity present is protected under the regulations and guidelines of the RAMSAR Convention, which seeks to promote the development of the territory, based on its ecological, botanical, zoological, limnological and hydrological conditions (Painii-Montero et al., 2020). Despite this designation, the Abras de Mantequilla wetland faces a series of pressures of human origin, including the extraction of water for irrigation, and consumption, deforestation, agricultural intensification and fishing exploitation (Quevedo, 2008; Alvarez-Mieles et al., 2013). These human activities are putting significant pressure on the biodiversity and overall health of the Abras de Mantequilla wetland. The ecosystem comprises permanent flooding lagoons, temporary flooding areas, and remnants of tropical dry forests. This area is located within the Southwestern Tropical zoogeographic zone (Irvine et al., 2022), and the predominant type of ecosystem is the flooded riparian grassland, with small remnants of coastal lowland deciduous forest (MAE, 2022). Furthermore, agricultural land use predominates in the area, where a forested area called “Noé Morán Forest” composed of a patch or remnant of forest with little human intervention and the presence of secondary succession forest species, is considered as a sustainable agroecosystem of the site; this area is approximately 10.6 hectares.
Abras de Mantequilla wetland faces a series of multifaceted challenges, including nutrient scarcity, water stress, erosion, phosphorus fixation, soil acidity, and soil biological diversity, which make soils especially susceptible to degradation and, there-fore, negatively affect the growth and development of plants (Cardoso & Kuyper, 2006; Qiu et al., 2019). Also, this Ecuadorian wetland system offers a range of ecosystem services such as flood regulation, nutrient retention, erosion control, and habitat provision (Portalanza et al., 2024). Gradual environmental dynamics significantly influence on the distribution of trees and associated organisms, such as mycorrhizal fungi. Arbuscular mycorrhizal fungi (AMF), whose hyphal network improves water absorption and essential minerals by colonized roots (Usman et al., 2021; Vieira et al., 2020). These fungi establish symbiotic relationships with approximately 80% of terrestrial vascular plants (Solís-Rodríguez et al., 2020), playing a crucial role in the establishment and maintenance of plant communities in forest ecosystems (Wang et al., 2019). Its effects on host plant physiology, including nutrient uptake from the root system and enhancement of soil carbon storage through the extraradical mycelium, provide an important pathway for the translocation of photosynthesis-derived carbon to microsites, which contributes to maintaining the soil structure (Rillig, 2004; Islam et al., 2022).
AMF also plays a key role in mobilizing nitrogen and phosphorus from organic polymers, releasing nutrients from mineral particles through weathe-ring, and mediating plant responses to stress factors such as drought, soil acidification, toxic metals and plant pathogens. Furthermore, they establish a variety of interactions with other soil micro-organisms, which contributes to improving soil fertility in terms of its physical, chemical and biological properties (Hartmann & Six, 2023). A series of physical, chemical, and biological indicators may be used to evaluate soil fertility. Physical indicators include soil texture, aggregation, moisture, porosity and bulk density, while chemical indicators include soil pH, total carbon and nitrogen, mineral nutrients, organic matter and soil cation exchange capacity. Biological indicators, such as microbial biomass, are also important to understand and monitor soil fertility.
The formation of symbiosis between fungi and plants in lightly disturbed environments, such as the “Noé Morán” secondary forest, is crucial due to the benefits that AMF provides to plants. These benefits include more significant absorption of essential nutrients, such as phosphorus, nitrogen, potassium and calcium, through external hyphal networks, facilitated by transporters such as H+-ATPase (Guzmán & Farías, 2005), as well as an increase in resistance against pathogenic agents, thanks to the ability of mycorrhizae to inhibit the growth of other competing microorganisms that could negatively affect plants (Ullah et al., 2024).
Currently, inadequate forest management and continuous loss of vegetation have led to adverse effects such as increased soil degradation and alteration of beneficial microflora and fauna in conventional agriculture. Faced with this problem, it is crucial to implement control measures, such as organic or ecological nutrition, to establish self-sustaining systems that take advantage of the ecosystem's natural responses, such as using green manures, humus, compost and beneficial micro-organisms. AMF are particularly relevant in this context, as they contribute to mobilizing and recycling nutrients to improve soil fertility. In this study, we sought to identify which AMF species are establishing beneficial symbioses with the most representative forest species of the "Noé Morán" secondary forest of the Abras de Mantequilla Wetland. This analysis allowed us better to under-stand the soil-plant-microorganism interactions in this sustainable agroecosystem.
2. Methodology
Study area and sampling design
This study was carried in the “Noé Morán” forest in the Abras de Mantequilla Wetland, Vinces Canton, Los Ríos Province, Ecuador. This wetland covers 56,000 ha and is in the province's central region (1° 28' 0" S; 79° 34' 59" W). In 2000, it was designated as a Ramsar site due to its importance a nesting area for various migratory bird species and its rich native ichthyological fauna. This wetland comprises natural depressions in the terrain that are fed mainly by water from rainfall, and the water supply from rivers and streams, with altitudes ranging between 5 and 10 meters (Quevedo, 2008).
The present investigation was carried out in the rainy season of 2023. Four the most numerous and representative forest tree species of the “Noé Morán” forest were selected, triplicate soil samples were collected in the vicinity of the Guazuma ulmifolia (Guasmo), Albizia guachapele (Guachapelí), Eugenia pustulescens (Mountain guayabo) and Cecropia peltata (Guarumo) at randomly established points within the forest, giving a total of 12 samples for the entire experiment. Each sample consisted of three subsamples taken within a one-meter radius of the main collection point, totaling approximately 2 kg of soil per sample. Collection was carried out using a Dutch auger (Efco) at a depth of 0-20 cm and the tool was meticulously cleaned with 98% alcohol to avoid contamination between each sampling. Soil samples were placed in previously identified and hermetically sealed plastic bags, then stored in cold boxes (20.7 L Electrolux) with ice. Subsequently, they were transferred to the Chemistry and Biochemistry laboratory of Universidad Técnica Estatal de Quevedo, Quevedo, Ecuador, where they were refrigerated at a temperature of 6 to 10 °C. After this, the processes of isolation, counting and identification of AMF spores were carried out, as well as the physicochemical analysis of the soil samples of the "Noé Morán" secondary forest.
Granulometric and chemical analysis of the soil
A portion of 300 cm3 of each soil sample was sent to the soil and water laboratory of the INIAP (National Agricultural Research Institute) Tropical Pichilingue Experimental Station, located at km 5 Vía Quevedo-El Empalme, Cantón Mocache, Province of Los Ríos, Ecuador to carry out the soil analyses. pipette method determined the soil texture (Teixeira et al., 2017). Soil pH was measured with distilled water (1:1), and P, K, Cu, Fe, Zn, and Mn were extracted in the Mehlich-1 solution. The elements Ca, Mg, and Al were extracted with a solution a (CH3 COO)2 CaH2O solution. Sulfur was extracted in a solution of SO4. The boron was extracted in hot water. Organic matter was evaluated by oxidation of soil samples with a sulfochromic solution and was determined using the Black Method (Teixeira et al., 2017).
Extraction and identification of AMF spores
AMF spores were extracted from 100 g of each soil sample by the wet sieving method (Gerdemann & Nicoloson, 1963) followed by centrifugation in a sucrose gradient (20% and 60%). Nested sieves (Fisher Scientific) with 200, 100, and 50 µm openings were used for wet sieving the 200 µm sieve was transferred to a Petri dish and inspected with a dissecting microscope to detect and collect spores and large sporocarps. The materials collected on the other sieves were transferred to Falcon tubes containing the sucrose gradient and centrifuged at 3000 rpm for 3 minutes. The supernatant from the centrifugation was decanted onto a 50-µm sieve, washed with sterile distilled water to remove excess sucrose, and transferred to Petri dishes for closer inspection of AMF spores under a dissecting microscope (Leica DM 2500). Phenotypic characteristics of the spores, such as size, color, shape and hyphal attachment, were used to distinguish the various morphotypes. Spores of each morphotype were permanently mounted on slides with polyvinyl-lactoglycerol (PVLG) and PVLG mixed with Melzer's reagent (1:1, v/v) and examined with a compound and camera mounted micros-cope (Leica DM 2500).
Spores were identified at the genus and species level by analyzing the spore wall structure, Melzer reaction, and other taxonomically informative features. For the AMF spore identification at the genus and species levels, comparisons were made with the original catalog of the species description, with those described in Blaszkowski (2012) and with online photos of reference crops from INVAM (http://invam.wvu.edu - West Virginia University, USA) to identify AMF spores at the genus and species level. Glomoid spores that could not be attributed to species were assigned to Glomus. The taxonomic classification followed as a reference is the one proposed by Redecker et al. (2013). The total abundance of AMF spores (per 100 g of soil) was obtained by counting the spores in each soil sample. The number of samples in which spores of a specific species were detected was used to calculate the frequency of occurrence (FO), expressed as a percentage. AMF species richness (S) was determined by counting fungal species per soil sample. The number of spores of each species was used as a measure of species abundance to calculate the Shannon-Wiener diversity index (H), and the relationship between the observed diversity and the expected maximum diversity among forest types was determined based on the species composition of AMF using the Pielou evenness index (J).
Statistical analysis
Data on total spore abundance were transformed using the log (x + 1) function before analysis, to meet the requirements of homogeneity of the variance (Levene's test) and the normality of the residuals (Kolmogorov-Smirnov test) for the datasets. Differences in total spore abundance and species richness between different locations were examined using a one-way analysis of variance (ANOVA), followed by a Tukey test (p < 0.05). All of these statistical analyzes were carried out using the VEGAN package within the R software environment (R Core Team, 2015).
Two matrices were obtained to correlate the compositions of the AMF communities with the physical and chemical properties of the soil. The first matrix was obtained with spore abundance for AMF species in each tree species type, and these data were subjected to the Hellinger transformation before analysis. Rare species (those comprising <10% of the total frequency) were removed from the analysis as the ordination power of the data decreased (Hill & Gauch, 1980). The second soil property matrix was obtained, and collinear variables were removed based on a variance inflation factor (VIF) ≥ 10. Both matrices were subjected to discriminant redundancy analysis (RDA) followed by a forward step of selection (Blanchet et al., 2008) to determine the proportion of AMF community variance explained by soil properties, followed by an ANOVA (p < 0.05). All multivariate analysis were performed using the “for” packages (Dray et al., 2016) and "vegan" (Oksanen et al., 2015) on the R software platform (R Core Team, 2015).
3. Results and discussion
Soil physicochemical properties
The set of physicochemical variables revealed relative differentiations according to the forest tree species in cultivation. The soil of the Albizia guachapele exhibited a predominance of clay, with a value of 167%, significantly surpassing the other cultivars. In this context, no alterations were observed in soil pH levels, which remained in a range between 5.46 and 6.07. Generally, certain forest species have the capacity to release organic acids through the decomposition of organic matter in the soil (Kupka & Gruba, 2022); these acids can acidify the soil and decrease its pH.
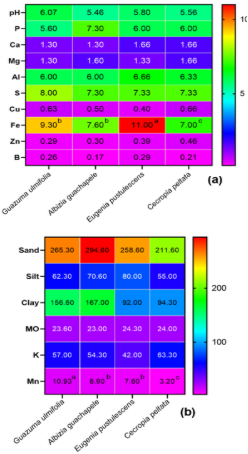
Figure 1. Physical and chemical analysis (a, b) of rhizosphere soils of four forest species from the “Butter wetlands”.
In addition, differences were identified in the iron content, with a value of 11 mg/kg in the soil with a predominance of Eugenia pustulescens, compared to the Cecropia peltata soil, which registered an average of 7 mg/kg. Several studies have shown that forest species have different nutrient requirements, including iron (Singh et al., 2021; Epihov et al., 2021). Some species can extract large amounts of iron from the soil to meet their growth needs, which can reduce the available iron content in the soil (Lambers et al., 2021).
Another important parameter that revealed outstanding values was the concentration of manganese (Mn), where the soil sample of Guazuma ulmifolia presented the highest values (Figures 1a and 1b), with an average of 10.93, in contrast to the lowest values obtained in the soil sample of Cecropia peltata tree, with an average of 3.26.
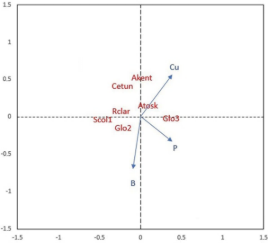
Figure 5. Redundancy Analysis (RDA) of the mycorrhizal species communities with soil parameters in the “Butter wetland”.
*AMF species abbreviation: Acaulospora kentinensis (Akent); Acaulospora koskei (Akosk); Claroideoglomus etunicatum (Cetun); Glomus sp2. (Glo2); Glomus sp3. (Glo3); Rhizophagus clarus (Rclar); Scutellospora sp1. (Scut1).
Composition of species/families in coverage
The composition analysis highlighted that the Albizia guachapele ecosystem exhibits a notable diversity of mycorrhizae associated with the root system, accounting for six identified species (Figure 4a-d). This reflects a significant level of symbiotic association in this tree species. A study carried out by Waring et al. (2016) highlighted that each plant species can have a unique relationship with certain species of mycorrhizal fungi. The ability of A. guachapele to interact and associate with a variety of mycorrhizae may be due to specific characteristics of its root system, root exudates or metabolites that favor this diversity (Blair et al., 2004; Balieiro et al., 2007).
Cecropia peltata mycorrhizal species, suggesting a healthy interaction with its underground environ-ment. Certain species may provide suitable habitat for a variety of mycorrhizae (Tedersoo et al., 2020; Huey et al., 2020). Less association of mycorrhizae in other plant species could be attributed to soil and environmental conditions that may not be optimal to sustain diversity (Janowski & Leski, 2022). Other factors such as nutrient availability, soil moisture and pH can influence the diversity and composition of the mycorrhizal community associated with C. peltata (Ma et al., 2021; Liu et al., 2021). On the other hand, the Eugenia pustulescens and Guazuma ulmifolia trees showed a moderate count of 3 and 2 mycorrhizal species, respectively, indicating lower diversity compared to A. guachapele and C. peltata.
Redundancy analysis
The redundancy analysis (RDA) performed reveals the relative contributions of various environmental variables to the variability in ecosystem composition (Figure 5). This approach allows discerning the specific influence of each environmental factor on plant community structure, providing a deeper understanding of the underlying ecological processes.
The redundancy analysis (RDA) is illustrated in Figure 5, highlighting the influence of specific soil parameters on the composition of arbuscular mycorrhizal fungi (AMF) communities within the ecosystem. The RDA results indicate that phosphorus (P), boron (B), and copper (Cu) have significant impacts on the variance in AMF composition among the sampled sites, as denoted by their p-values below 0.05. These nutrients play essential roles in plant-mycorrhizal interactions, with phosphorus and copper crucial for fungal metabolic processes and symbiotic efficiency (Shi et al., 2023; Bhupenchandra et al., 2024). In contrast, iron (Fe) and sulfur (S) did not reach statistical significance, suggesting, according to Zheng et al. (2024), that their concentrations may not substantially influence AMF community structure. This pattern underscores the selective role of certain soil nutrients in shaping AMF diversity and distribution, as forest species likely interact differently with AMF depending on soil nutrient availability.
4. Conclusions
This study highlights the diversity and function of arbuscular mycorrhizae (AMF) in the ecosystem of the "Abras de Mantequilla" wetland in Ecuador. Significant differences were observed in the physical-chemical properties of the soil between the tree species studied, as well as variations in the abundance and diversity of AMF species associated with each tree species. The findings highlighted the capacity of certain forest species, such as Albizia guachapele and Cecropia peltata, to favor the presence of AMF in the soil, which underlines the importance of conserving and adequately managing these natural areas to guarantee their ecological function and the sustainability of the system. This study underscores the role of certain forest species in enhancing AMF diversity in Ecuadorian wetlands. Future research should explore the application of AMF in reforestation and soil restoration, particularly in degraded areas, to support ecosystem resilience and soil health.
Acknowledgements
The Secretariat of Higher Education, Science, Technology and Innovation (SENESCYT, Ecuador) granted a scholarship to Jennifer Vivanco Ube at the Graduate School of the State Technical University of Quevedo (UTEQ) and provided the opportunity to study for a Master's degree in Agroecology and Sustainable Development. the UTEQ provided permits for the use of equipment and reagents in Biology and Microbiology laboratories.
Authors contribution
OPB: Writing – review & editing, Data curation, Funding acquisition. JVU: Writing – original draft, Funding acquisition, Investigation, Conceptualization. ACM: Writing – original draft, Formal analysis. JPUZ: Writing – review & editing, Software. NRM: Review & editing, Conceptualization. FRGF: Writing – review & editing.
Conflict of interest statement
The authors declare that they have no conflict of interest.
ORCID
O. O. Prieto-Benavides https://orcid.org/0000-0003-4101-0523
J. L. Vivanco-Ube https://orcid.org/0009-0007-8426-8854
A. V. Cedeño-Moreira https://orcid.org/0000-0002-6564-5569
J. P. Urdánigo https://orcid.org/0000-0002-8972-0279
N. R. Maddela https://orcid.org/0000-0002-7893-0844
F. R. Garcés-Fiallos https://orcid.org/0000-0002-1795-4439
References
Alvarez-Mieles, G., Irvine, K., Griensven, A. V., Arias-Hidalgo, M., Torres, A., & Mynett, A. E. (2013). Relationships between aquatic biotic communities and water quality in a tropical river-wetland system (Ecuador). Environmental Science and Policy, 34, 115–127. https://doi.org/10.1016/j.envsci.2013.01.011
Balieiro, F. D. C., Franco, A. A., Fontes, R. L. F., Dias, L. E., Campello, E. F. C., & Faria, S. M. D. (2007). Evaluation of the throughfall and stemflow nutrient contents in mixed and pure plantations of Acacia mangium, Pseudosamenea guachapele and Eucalyptus grandis. Revista Árvore, 31, 339–346. https://doi.org/10.1590/S0100-67622007000200017
Bennett, A. E., & Classen, A.T. (2020). Climate change influences mycorrhizal fungal–plant interactions, but conclusions are limited by geographical study bias. Ecology, 101(4), e02978. https://doi.org/10.1002/ecy.2978
Bhupenchandra, I., Chongtham, S. K., Devi, A. G., Dutta, P., Sahoo, M. R., et al. (2024). Unlocking the potential of arbuscular mycorrhizal fungi: exploring role in plant growth promotion, nutrient uptake mechanisms, biotic stress alleviation, and sustaining agricultural production systems. Journal of Plant Growth Regulation, 1–39. In press. https://doi.org/10.1007/s00344-024-11467-9
Blair, B. C., & Perfecto, I. (2004). Successional status and root foraging for phosphorus in seven tropical tree species. Canadian Journal of Forest Research, 34(5), 1128–1135. https://doi.org/10.1139/x04-004
Blanchet, F., Legendre, P., & Borcard, D. (2008) Forward selection of explanatory variables. Ecology, 89(9), 2623–2632. https://doi.org/10.1890/07-0986.1
Blaszkowski, J. (2012). Glomeromycota. W. Szafer Institute of Botany, Polish Academy of Sciences. Krakow
Cardoso, I. M., & Kuyper, T. W. (2006). Mycorrhizas and tropical soil fertility. Agriculture, Ecosystems & Environment, 116(1-2), 72–84. https://doi.org/10.1016/j.agee.2006.03.011
Carteron, A., Vellend, M., & Laliberte, E. (2022). Mycorrhizal dominance reduces local tree species diversity across US forests. Nature Ecology & Evolution, 6(4), 370–374. https://doi.org/10.1038/s41559-021-01634-6
Chakraborty, S. K., Sanyal, P., & Ray, R. (2023). Diversity and classification of wetlands in international and national perspectives. In Wetlands Ecology: Eco-biological uniqueness of a Ramsar site (East Kolkata Wetlands, India) (pp. 167-226). Cham: Springer International Publishing. https://doi.org/10.1007/978-3-031-09253-4_3
Deng, M., Hu, S., Guo, L., Jiang, L., Huang, Y., Schmid, B., et al. (2023). Tree mycorrhizal association types control biodiversity-productivity relationship in a subtropical forest. Science Advances, 9(3), eadd4468. https://doi.org/10.1126/sciadv.add4468
Dray, S., Legendre, P., Blanchet, G. (2016). Packfor: forward selection with permutation. R package version 0.0-8/r136. https://R-Forge.Rproject.org/projects/sedar/ accessed November 1, 2023
Epihov, D. Z., Saltonstall, K., Batterman, S. A., Hedin, L. O., Hall, J. S., van Breugel, M., et al. (2021). Legume–microbiome interactions unlock mineral nutrients in regrowing tropical forests. Proceedings of the National Academy of Sciences, 118(11), e2022241118. https://doi.org/10.1073/pnas.2022241118
Faghihinia, M., Zou, Y., Chen, Z., Bai, Y., Li, W., Marrs, R., & Staddon, P. L. (2020). Environmental drivers of grazing effects on arbuscular mycorrhizal fungi in grasslands. Applied Soil Ecology, 153, 103591. https://doi.org/10.1016/j.apsoil.2020.103591
Gerdemann, J., & Nicolson, T. (1963). Spores of mycorrhizal endogone species extracted from soil by wet sieving and decanting. Transactions of the British Mycological Society, 46(2), 235–244. https://doi.org/10.1016/S0007-1536(63)80079-0
Ghosh, S., Anju, P., Pattanayak, R., & Sahu, N. C. (2024). Fisheries and Aquaculture in Wetland Ecosystems: A Review of Benefits, Risks, and Future Prospects in India. Journal of Coastal Research, 40(3), 598–612. https://doi.org/10.2112/JCOASTRES-D-23-00045.1
Guzmán-González, S., & Farías-Larios, J. (2005). Biología y regulación molecular de la micorriza arbuscular. Avances en Investigación Agropecuaria, 9(2), 17–31.
Hartmann, M., & Six, J. (2023). Soil structure and microbiome functions in agroecosystems. Nature Reviews Earth & Environment, 4(1), 4–18. https://doi.org/10.1038/s43017-022-00366-w
Hill, M., & Gauch, H. (1980). Detrended correspondence analysis: An improved ordination technique. Vegetatio, 42, 47–58. https://doi.org/10.1007/BF00048870
Huey, C. J., Gopinath, S. C., Uda, M. N. A., Zulhaimi, H. I., Jaafar, M. N., Kasim, F. H., & Yaakub, A. R. W. (2020). Mycorrhiza: a natural resource assists plant growth under varied soil conditions. 3 Biotech, 10(5), 204. https://doi.org/10.1007/s13205-020-02188-3
Irvine, K., Dickens, C., Castello, L., Bredin, I., & Finlayson, C. (2022). Vegetated wetlands: from ecology to conservation management. Fundamentals of Tropical Freshwater Wetlands, 1, 589-639. https://doi.org/10.1016/B978-0-12-822362-8.00023-2
Islam, M., Al-Hashimi, A., Ayshasiddeka, M., Ali, H., & El Enshasy, H. (2022). Prevalence of mycorrhizae in host plants and rhizosphere soil: A biodiversity aspect. PloS One, 17(8), e0273463. https://doi.org/10.1371/journal.pone.0273463
Janowski, D., & Leski, T. (2022). Factors in the distribution of mycorrhizal and soil fungi. Diversity, 14(12), 1122. https://doi.org/10.3390/d14121122
Jerbi, M., Labidi, S., Lounes-Hadj Sahraoui, A., Chaar, H., & Ben Jeddi, F. (2020). Higher temperatures and lower annual rainfall do not restrict, directly or indirectly, the mycorrhizal colonization of barley (Hordeum vulgare L.) under rainfed conditions. PloS One, 15(11), e0241794. https://doi.org/10.1371/journal.pone.0241794
Kupka, D., & Gruba, P. (2022). Effect of pH on the sorption of dissolved organic carbon derived from six tree species in forest soils. Ecological Indicators, 140, 108975. https://doi.org/10.1016/j.ecolind.2022.108975
Lambers, H., Wright, I. J., Guilherme Pereira, C., Bellingham, P. J., Bentley, L. P., Boonman, A., et al. (2021). Leaf manganese concentrations as a tool to assess belowground plant functioning in phosphorus-impoverished environments. Plant and soil, 461, 43–61. https://doi.org/10.1007/s11104-020-04690-2
Liu, M., Shen, Y., Li, Q., Xiao, W., & Song, X. (2021). Arbuscular mycorrhizal fungal colonization and soil pH induced by nitrogen and phosphorus additions affects leaf C: N: P stoichiometry in Chinese fir (Cunninghamia lanceolata) forests. Plant and Soil, 461, 421–440. https://doi.org/10.1007/s11104-021-04831-1
Luo, Y. H., Ma, L. L., Seibold, S., Cadotte, M. W., Burgess, K. S., Tan, S. L., et al. (2023). The diversity of mycorrhiza‐associated fungi and trees shapes subtropical mountain forest ecosystem functioning. Journal of Biogeography, 50(4), 715–729. https://doi.org/10.1111/jbi.14563
Ma, X., Geng, Q., Zhang, H., Bian, C., Chen, HY, Jiang, D., & Xu, X. (2021). Global negative effects of nutrient enrichment on arbuscular mycorrhizal fungi, plant diversity and ecosystem multifunctionality. New Phytologist, 229(5), 2957–2969. https://doi.org/10.1111/nph.17077
MAE. (2022). Classification system of ecosystems of continental Ecuador. Undersecretariat of Natural Heritage, Ministry of the Environment of Ecuador, Quito.
Oksanen, J., Blanchet, G., Kindt, R., Legendre, P., Minchin, P., O'Hara, R., Simpson, G., Solymos, P., Stevens, M., & Wagner, H. (2015). Vegan: Community Ecology Package. R package version 2. 3-0. http://CRAN.R-project.org/package=vegan accessed November 4, 2023
Painii-Montero, V. F., Santillán-Muñoz, O., Barcos-Arias, M., Portalanza, D., Durigon, A., & Garcés-Fiallos, F. R. (2020). Towards indicators of sustainable development for soybeans productive units: a multicriteria perspective for the Ecuadorian coast. Ecological Indicators, 119, 106800. https://doi.org/10.1016/j.ecolind.2023.111405
Portalanza, D., Torres-Ulloa, M., Arias-Hidalgo, M., Piza, C., Villa-Cox, G., Garcés-Fiallos, F. R., Álava, E., Durigon, A., & Espinel, R. (2024). Ecosystem services valuation in the Abras de Mantequilla wetland system: A comprehensive analysis. Ecological Indicators, 158, 111405. https://doi.org/10.1016/j.ecolind.2023.111405
Quevedo, O. (2008). Ramsar File for the Abras de Mantequilla Wetland - Ecuador 2008. Guayaquil, Ecuador. Retrieved from http://suia.ambiente.gob.ec
Qiu, L., Bi, Y., jiang, B., Wang, Z., Zhang, Y., & Zhakypbek, Y. (2019). Arbuscular mycorrhizal fungi ameliorate the chemical properties and enzyme activities of rhizosphere soil in reclaimed mining subsidence in northwestern China. Journal of Arid Land, 11, 135–147. https://doi.org/10.1007/s40333-018-0019-9
R Core Team, (2015). A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna.
Redecker, D., Schüßler, A., Stockinger, H., Stürmer, S., Morton, J., Walker, C. (2013). An evidence-based consensus for the classification of arbuscular mycorrhizal fungi (Glomeromycota). Mycorrhiza, 23(7), 515–531. https://doi.org/10.1007/s00572-013-0486-y
Rillig, M. C. (2004). Arbuscular mycorrhizae, glomalin, and soil aggregation. Canadian Journal of Soil Science, 84(4), 355–363. https://doi.org/10.4141/S04-003
Rożek, K., Rola, K., Błaszkowski, J., Leski, T., & Zubek, S. (2020). How do monocultures of fourteen forest tree species affect arbuscular mycorrhizal fungi abundance and species richness and composition in soil. Forest Ecology and Management, 465, 118091. https://doi.org/10.1016/j.foreco.2020.118091
Shi, J., Wang, X., & Wang, E. (2023). Mycorrhizal symbiosis in plant growth and stress adaptation: from genes to ecosystems. Annual Review of Plant Biology, 74(1), 569–607. https://doi.org/10.1146/annurev-arplant-061722-090342
Singavarapu, B., Beugnon, R., Bruelheide, H., Cesarz, S., Du, J., Eisenhauer, N., et al. (2022). Tree mycorrhizal type and tree diversity shape the forest soil microbiota. Environmental Microbiology, 24(9), 4236–4255. https://doi.org/10.1111/1462-2920.15690
Singh, D., Sillu, D., Kumar, A., & Agnihotri, S. (2021). Dual nanozyme characteristics of iron oxide nanoparticles alleviate salinity stress and promote the growth of an agroforestry tree, Eucalyptus tereticornis Sm. Environmental Science: Nano, 8(5), 1308–1325. https://doi.org/10.1039/D1EN00040C
Solís-Rodríguez, U. R., Ramos-Zapata, J.A., Ramos-Zapata, J. A., Hernández-Cuevas, L., & Salinas- Peba, L. (2020). Arbuscular mycorrhizal fungi diversity and distribution in tropical low flooding forests in Mexico. Mycological Progress, 19, 195–204. https://doi.org/10.1007/s11557-019-01550-x
Tedersoo, L., Bahram, M., & Zobel, M. (2020). How mycorrhizal associations drive plant population and community biology. Science, 367(6480), eaba1223. https://doi.org/10.1126/science.aba1223
Teixeira, P., Donagemma, G., Fontana, A., Teixeira, W. (2017). Manual de métodos de análise de solo. Embrapa Solos, Brasilia. 574 p.
Ullah, A., Gao, D., & Wu, F. (2024). Common mycorrhizal network: the predominant socialist and capitalist responses of possible plant–plant and plant–microbe interactions for sustainable agriculture. Frontiers in Microbiology, 15, 1183024. https://doi.org/10.3389/fmicb.2024.1183024
Usman, M., Ho- Plágaro, T., Frank, H., Calvo-Polanco, M., Gaillard, I., Garcia, K., & Zimmermann, D. (2021). Mycorrhizal symbiosis for better adaptation of trees to abiotic stress caused by climate change in temperate and boreal forests. Frontiers in Forests and Global Change, 4, 742392 https://doi.org/10.3389/ffgc.2021.742392
Vieira, L. C., Silva, D. K., Escobar, I. E., Silva, J. M., Moura, I. A., Oehl, F., & Silva, G. A. (2020). Changes in an arbuscular mycorrhizal fungi community along an environmental gradient. Plants, 9(1), 52. https://doi.org/10.3390/plants9010052
Wang, J., Wang, G. G., Zhang, B., Yuan, Z., & Fu, Z. (2019). Arbuscular mycorrhizal fungi associated with tree species in a planted forest of Eastern China. Forests, 10(5), 424. https://doi.org/10.3390/f10050424
Waring, B. G., Gei, M. G., Rosenthal, L., & Powers, J. S. (2016). Plant–microbe interactions along a gradient of soil fertility in tropical dry forest. Journal of Tropical Ecology, 32(4), 314–323. https://doi.org/10.1017/S0266467416000286
Zhang, J., Quan, C., Ma, L., Chu, G., Liu, Z., & Tang, X. (2021). Plant community and soil properties drive arbuscular mycorrhizal fungal diversity: A case study in tropical forests. Soil Ecology Letters, 3, 52–62. https://doi.org/10.1007/s42832-020-0049-z
Zhen, L. I., Songlin, W. U., Yunjia, L. I. U., Qing, Y. I., Merinda, H. A. L. L., Narottam, S. A. H. A., et al. (2024). Arbuscular mycorrhizal fungi regulate plant mineral nutrient uptake and partitioning in iron ore tailings undergoing eco-engineered pedogenesis. Pedosphere, 34(2), 385–398. https://doi.org/10.1016/j.pedsph.2023.04.004