1. Introducción
Tara (Caesalpina spinosa) is a tree of Andean origin widely cultivated in Peru. This legume grows at an altitude of between 500 and 3,200 m in places with moderate rainfall and temperatures between 12 and 24 °C (De La Torre, 2018). The industry's interest in this tree lies in the use of its fruits, which are flattened and indehiscent pods of orange color and a size of 8 to 10 cm long and 2 cm wide (Pino et al., 2013). In the pods, there are round dark brown seeds, which represent 38% of the pod. Already, the ground seedless pods (tara powder) are mainly used in the tanning, alcohol, paints, pharmacy, and cosmetics industries (De La Torre, 2018).
The main product of Tara's industrial interest is in the seed's endosperm, which is Tara gum. A food hydrocolloid (E417) is highly sought for its rheolo-gical characteristics, generating high viscosity and thickening power at low concentrations. This behavior is due to its chemical structure of the galactomannan type, which is very similar to locusta gum and has technological properties similar to xanthan gum (Ahmad et al., 2019). This gum has been widely studied in recent years, such as a stabilizer for emulsions (Vélez-Erazo et al., 2020), gels (Ingrassia et al., 2019; Zhang et al., 2019), gluten-free bread (Vidaurre-Ruiz et al., 2019), and biofilms (Liu et al., 2020), among other applications.
Additionally, the seed germ is rich in protein content (Fierro et al., 2024), generating a new protein source as a by-product of the processing of this tree in the production of its gum. Notably, there needs to be more information found in the literature based on this protein source, generating interest in studying its functional properties, such as water or oil retention and the ability to form foams, gels, or emulsions.
On the other hand, the sacha inchi (Plukenetia volubilis L.) is also mainly native to the Peruvian jungle, growing in tropical forests between 200 to 1500 m altitude, with temperatures between 10 to 37 °C and with rainfall between 850 to 1000 mm al year (Goyal et al., 2022). Oil is the main product of this plant, which has generated great interest in the international market due to its chemical composition and potential health benefits. In the seed, the oil is found in a concentration between 41% - 54% (Otálora et al., 2020), mainly composed of mono- and polyunsaturated fatty acids (MUFA and PUFA, respectively). Silva et al. (2019) report that sacha inchi oil is mainly composed of oleic acid (14.65%), linoleic acid (42.5%), and linoleic acid (30.75%). The presence of high concentrations of the latter fatty acid is what makes sacha inchi oil so desirable on the market. It also contains micronutrients such as phytosterols, tocopherols, and phenolic compounds (Fanali et al., 2011), which is why its consumption is beneficial for health as it helps in the control of diseases such as obesity, diabetes, coronary and neurodegenerative diseases.
However, these types of oils are susceptible to oxidative deterioration, which generates unpleasant odors and flavors (Augustin et al., 2006). Additionally, it is chemically incompatible when incorporated into applications related to aqueous systems. Microencapsulation can be used to avoid or delay this process and transport the oil in aqueous systems. This technology protects different food or functional components, such as fatty acids, from various processing and storage factors, such as light, air, or humidity. It also masks taste, aroma, and unpleasant flavors while maintaining quality (Alarcón et al., 2020; Choudhury et al., 2021). Currently, research has been reported on obtaining stable emulsions of sacha inchi oil using different emulsifiers and stabilizers (Silva et al., 2019; Vicente et al., 2018).
In the oils microencapsulation, one of the most critical processes is emulsification, where the use of ultrasound has aroused great interest in recent years because the good stability of the emulsion is related to the size of the drop formed, representing a critical parameter in encapsulation efficiency (Rodea-González et al., 2012). For this reason, along with the technology selection, wall materials that are suitable for the design of emulsions must be chosen.
Proteins have good emulsifying properties that allow them to stabilize and prevent the lipid and aqueous phases from separating (Chang & Nickerson, 2018). In this sense, currently, vegetable proteins (e.g., oilseeds, legumes, and cereals) are being used more as emulsifying material because they have a lower degree of allergens, there is also a wide variety of sources, and they have a lower cost (Nesterenko et al., 2013). This is the case of Tara. However, until now, no research has been reported on using tara protein (CTP) as a stabilizer in emulsion formation.
For all the above, the objective of this research was to characterize the functional properties of the commercial tara protein or tara seed germ, studying its ability to obtain sacha inchi oil emulsions through ultrasound in more depth.
2. Methodology
2.1. Materials
Sacha inchi oil was obtained from Shanantina (Lamas, San Martin, Peru) and commercial tara protein (CTP) was obtained from tara germ and was purchase from Molinos Asociados SAC (Lima, Peru). Distilled water was used in all analysis.
2.2. Sample characterization
Commercial tara protein was characterized in terms of water content, ashes, lipids and protein content by the methods 966.02, 923.03, 920.39 and 920.87 of the Association of Official Analytical Chemists (AOAC) (AOAC, 2006). Carbohydrates were quantified by subtraction.
2.3. Functional properties
Water and oil absorption capacities
0.25 g of CTP was placed in a 50 mL centrifuge tube. Subsequently, 10 mL of distilled water for water absorption capacity (WAC) or sacha inchi oil for oil absorption capacity (OAC) was added. Centrifuge tubes were shake in a vortex (Heidolph, Germany) for 10 s every 5 min for 30 min. After the stirring time, samples were centrifuged (Centrifuge 5702, Eppendorf, Germany), at 1000 g for 15 min. The supernatant was then separated, and the tube was weighed along. For the OAC case, the tube was placed upside down for 10 min before weighing it. WAC and OAC were calculated according to equation 1 and were expressed in g of absorbed water/g of dry matter and g of absorbed oil/g of dry matter (Stone et al., 2015).
 [1]
[1]
Foam stability
CTP solutions were prepared at 0.05%, 1.0% and 2.0% (w/w) and hydrated for 2 hours in a magnetic stirrer (C-MAG HS 7, IKA, Germany). Then, 25 mL of the solutions were placed in 50 mL centrifuge tubes and homogenized in a Ultraturrax® (IKA®-Werke GmbH & Co. KG, Germany) at 8,000 rpm for 5 min. Subsequently, the solution was transferred to 50 mL graduated cylinders. Foam volume was measured immediately after being placed in the test tube and after 30 min. Foam stability (FS) were carried out through equation 2 (Lam et al., 2017).
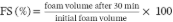 [2]
[2]
Gelling properties
Boye et al. (2010) method was used with modifi-cations. 20 g dispersions of CTP were prepared at 3%, 6%, 9%, 12%, 15%, 18% and 21%, which were stirred (C-MAG HS 7 magnetic stirrer, IKA, Ger-many) for 1 hour to obtain a homogeneous mixture. Then, 5 g of the dispersion were weighed into 15 mL falcon tubes and subsequently placed in a water bath (Aqualine AL 18, LAUDA, Germany) at 90 ºC for 30 minutes. Once the time was up, samples were cooled in an ice bath for a period of 5 minutes. The least gelation concentration was determined when a semi-solid remained adhered to the bottom of the tube.
2.4. Emulsifying properties
Preparation of protein solution
CTP dispersions were prepared at 2%, 4% and 6% (w/w) and stirred for 20 hours to ensure hydration (C-MAG HS 7 magnetic stirrer, IKA). Samples were placed in falcon tubes to be centrifuged at 4000 rpm for 15 minutes (Centrifuge 5702, Eppendorf, Germany). Supernatant was removed and placed in beakers to form emulsions.
Coarse emulsion (RS emulsions)
To prepare the RS emulsions, the method of Vélez-Erazo et al. (2018) was followed with modifications. Oil in water emulsions were formed using the previously prepared CTP solutions with sacha inchi oil (SIO) at 15%, 20% and 25%. Emulsions were homogenized with rotor-stator device Ultraturrax® (IKA®-Werke GmbH & Co. KG, Germany) at 8,000 rpm while the oil was added in the form of a thread; later, the speed was increased to 12,000 rpm for a period of 5 minutes.
Final emulsions (RS-US emulsions)
75% of the RS emulsion was mixed with 25% of tara gum dispersion (2.0% w/w), and the RS-US emulsion was subjected to sonication during 3 min at 75% of power amplitude (Ultrasound, Branson 250, USA, probe (Ø = 12.5 mm)). After obtaining both types of emulsions (RS and RS-US), the respective characterizations were carried out.
2.5. Emulsions characterization
Emulsion stability
Creaming index (CI) was evaluated as described by Taha et al. (2018) with certain modifications. Emulsions were placed in graduated cylinders of 50 and 100 mL, subsequently sealed and kept at room temperature. CI was calculated by time periods (30 min, 1, 4 and 24 hours) using equation 3 (CI: Creaming index, Vo: Initial emulsion, V: Volume of the upper phase).
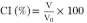 [3]
[3]
Optical microscopy
Emulsion drops were placed on a glass slide and covered with coverslips. After this, their microstructure was observed under a microscope (Eclipse E200, Nikon Instruments Inc., USA), at 40x of magnification. The images were taken by the camera integrated into the microscope using the Motic Images Plus 2.0 software.
Droplet size
With the help of ImageJ software, the area (S) of 400 drops was measured and subsequently the cal-culation of the theoretical average diameter (D[Th]) was carried out with equation 4 (Saout et al., 1999).
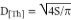 [4]
[4]
2.6. Statistical analysis
All experiments had 2 experimental and 3 analytical repetitions, which were evaluated by analysis of variance (ANOVA). The results obtained were statistically analyzed using Minitab software (Minitab 17, United States). Next, the averages were compared using the Tukey test; for differences, significance was considered at 5%.
3. Results and discussion
3.1. Proximal composition
The proximal composition of CTP is presented in Table 1. Moisture, fat, protein and ash represented 6.03%, 12.32%, 45.16%, and 6.04% respectively. These values are similar to those reported in previous data (Del Re-Jimenez & Amado, 1989; Fierro et al., 2024), who found a humidity between 4.2% - 6.24%, a fat content between 13.5% - 13.93%, protein content between 41.6% – 54.32%, and ash content of 6.30%.
When compared with carob germ flour, another legume used to produce locust bean gum, it is observed that it has more protein than CTP (59.5%), but less fat, while moisture and ash are similar (Dakia et al., 2007). On the other hand, Silva et al. (2015) reported for pajuro seed flour, a species belonging to the Fabaceae family like tara, from different regions, lower values than those reported for tara, with protein ranging between 21.7 and 24.2%, a humidity of 9.47 and 9.13%, fat 3.01 and 2.71%, and ashes 2.69 and 2.71%.
Table 1
Proximal composition of Commercial Tara Protein (CTP)
Compound | Values |
Moisture (%) | 6.03 ± 0.23 |
Protein (%) | 45.16 ± 0.06 |
Lipids (%) | 12.32 ± 0.03 |
Ash (%) | 6.04 ± 0.19 |
Carbohydrates (%)a | 36.48 ± 0.52 |
Crude fiber (%) | 2.49 ± 0.02 |
a Carbohydrate content was determined by difference from total composition.
3.2. Functional properties
3.2.1. Water and oil absorption capacities
Water and oil absorption capacities were evaluated after decantation of water and oil that was absorbed, where the results indicated that the CTP absorbed 2.14 ±0.26 g of oil for each gram of dry matter, while it absorbed 1.82±0.02 g of water for each gram of dry matter. This indicates that the protein flour has a greater affinity for oil than for water. Lafarga et al. (2018) reported 2.33 ± 0.12 and 2.69 ± 0.32 g of water or oil per g of isolated Ganxet bean protein concentrate, respectively. The value obtained in OAC is similar, but in the case of WAC it is higher than that found in the tara protein. On the other hand, the values obtained in this work are higher than those reported for red lentils with 1.90 ± 0.01 and 1.23 ± 0.01 g of water or oil respectively (Stone et al., 2019). Since this protein flour is marketed for use in the food sector, these two properties are of great importance, because both are indicators for determining whether its addition and use in different food products is feasible (Santana et al., 2017).
3.2.2. Foam stability
To analyze the foaming property of tara protein flour, three different percentages were used: 0.05%, 1.0% and 2.0%. Foaming was not observed in any of these percentages, indicating that tara protein flour does not have this capacity. Foam formation depends on several factors, such as concentration, protein solubility, the presence of lipids, and the degree of protein denaturation. In this case, two factors were identified that could have prevented foam formation: the high percentage of fat that this protein flour has (Table 1) and the low concentrations used in the analyzes, since the optimal concentrations for the formation of foam in proteins range between 2% and 8% (Damodaran, 1996; Santos Teixeira, 2009).
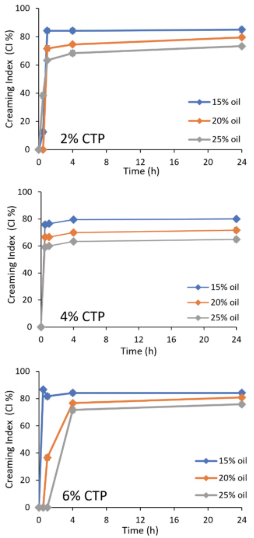
Figure 1. Creaming index of rotor-stator (RS) emulsions stabilized by commercial tara protein (CTP) at different CTP and chia oil concentrations.
3.2.3. Gelling properties
In this work, the gelation capacity of tara protein flour at different concentrations was evaluated. It was observed that the least gelation concentrations were 18% and 21%, which formed consistent and strong gels. On the contrary, concentrations of 3%, 6% and 9% failed to form gels, while those at 12% and 15% formed weak and viscous gels. These results agree with those reported by Dakhili et al. (2019), who indicated that the formation of gels depends on several factors such as heat, pH and enzymatic action.
On the other hand, Cajanus Cajan L. Millsp is a legume with high protein content. García et al. (2012) found that Cajanus Cajan L. Millsp flour required a minimum gelation concentration of 10%, lower than that of CTP. This difference could be due to the method of obtaining the flours, since the tara protein flour was obtained only by selection and grinding, while the Cajanus Cajan L. Millsp flour was subjected to a previous cooking process at 98 ºC followed by drying and grinding.
3.3. Coarse emulsion (RS emulsion) characterization
3.3.1. Emulsion stability
After evaluating the emulsions for the established time, the creaming index (CI) was calculated, and the results are shown in Figure 1 and Table 2. Table 2 shows the four-hour CI; this time was chosen to present the mean values of CI after the most stable emulsion became unstable. It was observed that all RS emulsions were unstable for a short period of time. Emulsions with 2% and 4% CTP had the highest CI in the first hour of evaluation, showing phase separation after 30 minutes. On the other hand, the CI decreased with increasing oil percen-tages. Only the emulsions with 6% protein emulsion and 25% oil remained stable for 1hour.
Table 2
Creaming index (CI) at 4h of stability and Mean droplet size (D[th]) of RS emulsions
CTP | Oil | CI at 4h (%) | (D[th]) (µm) |
2% | 15% | 84.17 ± 1.67 Aa | 8.68 ± 5.88 Bc |
20% | 74.58 ± 0.83 Bb | 13.14 ± 10.34 Ba |
25% | 68.33 ± 1.93 Bc | 10.59 ± 9.36 Bb |
4% | 15% | 79.58 ± 0.83 Ba | 8.51 ± 6.53 Bb |
20% | 70.00 ± 0.00 Cb | 10.96 ± 8.35 Ca |
25% | 63.35 ± 0.00 Cc | 8.81 ± 7.28 Cb |
6% | 15% | 84.17 ± 1.67 Aa | 12.92 ± 12.43 Ab |
20% | 76.67 ± 0.00 Ab | 15.90 ± 11.38 Aa |
25% | 71.67 ± 1.93 Ac | 12.29 ± 12.79 Ab |
CTP: commercial tara protein. RS: rotor-stator. Different letters indicate significant differences (p < 0.05). Capital letters: differentiate different percentages of protein. Lowercase letters: difference between different percentages of oil.
Some authors report low emulsion stability when formed only by the rotor-stator. Gomes and Kurozawa (2020) reported the formation of a creaming phase during the first hours of storage of emulsions stabilized by hydrolyzed rice protein. It is known that this method of emulsion formation produces systems with large droplet sizes, which promotes flocculation and subsequent coalescence of the droplets.
The results indicated that the emulsions with 15% oil had the smallest droplets (between 6.87 and 9.16 μm). However, the size distribution of emulsion with 15% oil presented the lowest %volume, and especially, at 2% CPT, a broad size distribution (Figure 4).
Table 3
Creaming index (CI) at 4h and Mean droplet size (D[th]) of RS-US emulsions
CTP | Oil | CI at 4h (%) | (D[th]) (µm) |
2% | 15% | 2.03 ± 0.06 Ca | 7.24 ± 5.18 Bb |
20% | 1.50 ± 1.92 Ba | 7.36 ± 2.68Cb |
25% | 0.00 ± 0.00 Ba | 8.15 ± 3.64 Ca |
4% | 15% | 18.00 ± 5.42 Ba | 6.87 ± 2.30 Bb |
20% | 2.00 ± 0.00 Bb | 9.01 ± 3.68 Ba |
25% | 0.00 ± 0.00 Bb | 9.77 ± 12.62 Ba |
6% | 15% | 64.00 ± 1.63 Aa | 9.16 ± 5.01 Ab |
20% | 49.00 ± 1.16 Ab | 11.27 ± 4.07 Aa |
25% | 40.00 ± 4.90 Ac | 11.95 ± 6.43 Aa |
CTP: commercial tara protein. RS-US: rotor-stator + ultrasound. Different letters indicate significant differences (p < 0.05). Capital letters: differentiate different percentages of protein. Lowercase letters: difference between different percentages of oil.
This same behavior was observed when comparing 2% and 4% CTP. These CTP concentrations presented the lowest droplet size (6.87 and 9.77 μm). In contrast, the emulsions with 6% presented larger and more heterogeneous droplets, with ranges from 9.16 to 11.95 μm. These findings were confirmed with microscopic images (Fig 4), which revealed, in fact, the decrease in emulsions' droplet size, the homogenization of size, and the oil incorporation because no free oil was observed in micrographs.
Alcântara et al. (2019) found values of 3.45 and 4.62 μm for emulsions prepared with chia oil and maltodextrin, whey protein isolate, and gum Arabic with the rotor-stator method, and diameters between 0.52 and 0.86 μm for rotor-stator/ ultrasound method. Likewise, in producing mono and double-layer emulsions of chia oil, the diameter found ranged between 0.93 to 1.44 μm using only ultrasound (Vélez-Erazo et al., 2018). These two investigations coincide and support the results obtained in this work by reducing the droplet size by subjecting the emulsions to the high energy capacity provided by ultrasound, which generates greater shearing of the dispersed droplets, reducing the size.
Obtaining small drop sizes was not necessarily related to the stability of the emulsions since, although the smallest drop sizes were observed at 15%, the most unstable emulsions were also ob-tained, especially at the lowest CTP concentration. In this case, an increase in the percentage of oil in the system (20% and 25%) could have influenced the viscosity of the emulsion and, consequently, could have decreased the mobility of the droplets (Vélez-Erazo et al., 2020).
The joint action between the decrease in size with the application of ultrasound, the steric effect with the addition of tara gum in the continuous phase, and an adequate concentration of CTP that covered the drops' surface allowed the stable systems obtention for 4 hours with very low or no phase separation. These observations are in agreement with Sun et al. (2019) and Zhou et al. (2021) who mentioned that several factors can intervene in the stability of emulsions, such as the concentration of the interfacial protein, the distribution of the droplets, the rheological properties, and the zeta potential.
Shao & Tang (2014) showed that the higher the concentration of soy protein in the emulsions, the more stable they were, substantially reducing the creaming index. On the other hand, in this work, we observed that the higher the percentage of CTP, the less stable the emulsions were, whether with the rotor-stator alone or with the rotor-stator/ ultrasound.
Likewise, tara gum contributed to RS-US emulsions having more stability. As mentioned by Vélez-Erazo et al. (2020), this polysaccharide has a high thickening capacity that reduces the mobility and coalescence of the droplets, keeping the size of the initially generated droplets stable. Also, it can be inferred that the emulsions with 6% CTP have large particles other than protein (the sample is not an isolated protein) that could not be solubilized in the CTP solution. This means there could probably be a chemical rejection with the other components, which causes a separation of the phases after 30 minutes of evaluation.
4. Conclusions
The commercial tara protein (CTP) presented a considerable protein content (45.16%), which allows it to be considered for studies of its functional properties. It was also observed that CTP had a greater oil absorption capacity than water absorption and did not present any foaming capacity. Finally, CTP has a minimum gelation concentration of 18%. Regarding the emulsifying properties, the RS-US emulsions (Rotor-stator- Ultrasound - tara gum), prepared with 25% sacha inchi oil and CTP concentrations of 2% and 4%, presented the best stability against the creaminess, remaining stable for at least 4 hours despite not having the smallest drop sizes. Future studies are necessary to deepen the use of this residue since, as shown in this work, it may have promising functional properties. By obtaining stable emulsions for at least 4 hours, this material could be used in applications that require stability for this time. Future research is also necessary to isolate the tara protein and evaluate whether it could improve the emulsions' stability in its purest form.
Acknowledgments
The authors are grateful to the Instituto de Investigación – UNSM for the financial support according to Resolución 623-2022-UNSM/CU-R and Resolución 611-2022-UNSM/CU-R. Additionally, the authors thank Professor Mike Corazón Guivin for access to the microscope.
Statements and Declarations
Conflict of interest
None of the authors has any conflict of interest in this research.
Author Statement
Sulca-Vásquez J. A.: conceptualization, formal analysis, investi-gation, visualization, Writing – original draft. Vélez-Erazo E. M.: Conceptualization, funding acquisition, methodology, project administration, resources, supervision, Writing – original draft, Writing – review & editing. Pasquel-Reátegui J. L.: project adminis-tration, Writing – review & editing, visualization. Mendieta-Taboada O. M.: supervision, Writing – review & editing.
ORCID
J.A. Sulca-Vásquez https://orcid.org/0000-0002-7463-7624
E. M. Vélez-Erazo https://orcid.org/0000-0002-8632-5329
J. L. Pasquel-Reátegui https://orcid.org/0000-0001-6467-394X
O. M. Mendieta-Taboada https://orcid.org/0000-0003-4302-6852
References
Ahmad, S., Ahmad, M., Manzoor, K., Purwar, R., & Ikram, S. (2019). A review on latest innovations in natural gums based hydrogels: Preparations & applications. International Journal of Biological Macromolecules, 136, 870–890. https://doi.org/10.1016/j.ijbiomac.2019.06.113
Alarcón, R., Gonzales, B., Sotelo, A., Gallardo, G., Pérez-Camino, M. C., & Chasquibol, N. (2020). Microencapsulation of Sacha Inchi (Plukenetia huayllabambana) Oil by Spray Drying with Camu Camu (Myrciaria dubia (H.B.K.) Mc Vaugh) and Mango (Mangifera indica) Skins. Proceedings, 53(1), 11. https://doi.org/10.3390/proceedings2020053011
Alcântara, M. A., Lima, A. E. A. de, Braga, A. L. M., Tonon, R. V., Galdeano, M. C., Mattos, M. da C., Brígida, A. I. S., Rosenhaim, R., Santos, N. A. dos, & Cordeiro, A. M. T. de M. (2019). Influence of the emulsion homogenization method on the stability of chia oil microencapsulated by spray drying. Powder Technology, 354, 877–885. https://doi.org/10.1016/j.powtec.2019.06.026
AOAC. (2006). Official Methodos of Analisis (Association of Official Analytical Chemist (ed.); Vol 18). Association of Official Analytical Chemist.
Augustin, M. A., Sanguansri, L., & Bode, O. (2006). Maillard Reaction Products as Encapsulants for Fish Oil Powders. Journal of Food Science, 71(2), E25–E32. https://doi.org/10.1111/j.1365-2621.2006.tb08893.x
Boye, J. I., Aksay, S., Roufik, S., Ribéreau, S., Mondor, M., Farnworth, E., & Rajamohamed, S. H. (2010). Comparison of the functional properties of pea, chickpea and lentil protein concentrates processed using ultrafiltration and isoelectric precipitation techniques. Food Research International, 43(2), 537–546. https://doi.org/10.1016/j.foodres.2009.07.021
Chang, C., & Nickerson, M. T. (2018). Encapsulation of omega 3-6-9 fatty acids-rich oils using protein-based emulsions with spray drying. Journal of Food Science and Technology, 55(8), 2850–2861. https://doi.org/10.1007/s13197-018-3257-0
Cho, Y. H., & McClements, D. J. (2009). Theoretical stability maps for guiding preparation of emulsions stabilized by protein-polysaccharide interfacial complexes. Langmuir, 25(12), 6649–6657. https://doi.org/10.1021/la8006684
Choudhury, N., Meghwal, M., & Das, K. (2021). Microencapsulation: An overview on concepts, methods, properties and applications in foods. Food Frontiers, May, 1–17. https://doi.org/10.1002/fft2.94
Dakhili, S., Abdolalizadeh, L., Hosseini, S. M., Shojaee-Aliabadi, S., & Mirmoghtadaie, L. (2019). Quinoa protein: Composition, structure and functional properties. Food Chemistry, 299, 125161. https://doi.org/10.1016/j.foodchem.2019.125161
Dakia, P. A., Wathelet, B., & Paquot, M. (2007). Isolation and chemical evaluation of carob (Ceratonia siliqua L.) seed germ. Food Chemistry, 102, 1368–1374. https://doi.org/10.1016/j.foodchem.2006.05.059
Damodaran, S. (1996). Amino Acids, Peptides, and Proteins. In Food Chemistry (3rd ed, pp. 1–370). MARCEL DEKKER, INC.
De La Torre, L. (2018). La Tara beneficios ambientales y recomendaciones para su manejo sostenible en relictos de bosque y sistemas agroforestales. In Condesan. https://condesan.org/wp-content/uploads/2018/10/Libro-Tara-Condesan-2.pdf
Del Re-Jimenez, B. L., & Amado, R. (1989). Comparative study of the chemical composition of germ meals from carob, guar and tara seeds. Food Hydrocolloids, 3(2), 149–156. https://doi.org/10.1016/S0268-005X(89)80024-4
Fanali, C., Dugo, L., Cacciola, F., Beccaria, M., Grasso, S., Dachà, M., Dugo, P., & Mondello, L. (2011). Chemical characterization of Sacha inchi (Plukenetia volubilis L.) oil. Journal of Agricultural and Food Chemistry, 59(24), 13043–13049. https://doi.org/10.1021/jf203184y
Fierro, O., Siano, F., Bianco, M., Vasca, E., & Picariello, G. (2024). Comprehensive molecular level characterization of protein-and polyphenol-rich tara (Caesalpinia spinosa) seed germ flour suggests novel hypothesis about possible accidental hazards. Food Research International, 181, 114119. https://doi.org/10.1016/j.foodres.2024.114119
García, O., Aiello, C., Peña, M. C., Ruíz, J. L., & Acevedo, I. D. C. (2012). Caracterización físico quimicas y propiedades funcionales de la harina obtenida de granos de quinchoncho (Cajanus cajan (L.) Millsp.) sometidos a diferentes procesamientos. Revista Cientifica UDO Agrícola, 12(4), 919–928.
Gomes, M. H. G., & Kurozawa, L. E. (2020). Improvement of the functional and antioxidant properties of rice protein by enzymatic hydrolysis for the microencapsulation of linseed oil. Journal of Food Engineering, 267(June 2019), 109761. https://doi.org/10.1016/j.jfoodeng.2019.109761
Goyal, A., Tanwar, B., Kumar Sihag, M., & Sharma, V. (2022). Sacha inchi (Plukenetia volubilis L.): An emerging source of nutrients, omega-3 fatty acid and phytochemicals. Food Chemistry, 373(PB), 131459. https://doi.org/10.1016/j.foodchem.2021.131459
Ingrassia, R., Bea, L. L., Hidalgo, M. E., & Risso, P. H. (2019). Microstructural and textural characteristics of soy protein isolate and tara gum cold-set gels. LWT - Food Science and Technology, 113, 108286. https://doi.org/10.1016/j.lwt.2019.108286
Lafarga, T., Álvarez, C., Bobo, G., & Aguiló-Aguayo, I. (2018). Characterization of functional properties of proteins from Ganxet beans (Phaseolus vulgaris L. var. Ganxet) isolated using an ultrasound-assisted methodology. LWT - Food Science and Technology, 98, 106–112 Contents. https://doi.org/10.1016/j.lwt.2018.08.033
Lam, A. C. Y., Warkentin, T. D., Tyler, R. T., & Nickerson, M. T. (2017). Physicochemical and functional properties of protein isolates obtained from several pea cultivars. Cereal Chemistry, 94(1), 89–97. https://doi.org/10.1094/CCHEM-04-16-0097-FI
Liu, F., Chang, W., Chen, M., Xu, F., Ma, J., & Zhong, F. (2020). Film-forming properties of guar gum, tara gum and locust bean gum. Food Hydrocolloids, 98, 105007. https://doi.org/10.1016/J.FOODHYD.2019.03.028
Nesterenko, A., Alric, I., & Durrieu, V. (2013). Vegetable proteins in microencapsulation: A review of recent interventions and their effectiveness. Industrial Crops and Products, 42, 469–479. https://doi.org/10.1016/j.indcrop.2012.06.035
Otálora, M. C., Camelo, R., Wilches-Torres, A., Cárdenas-Chaparro, A., & Gómez Castaño, J. A. (2020). Encapsulation Effect on the In Vitro Bioaccessibility of Sacha Inchi Oil (Plukenetia volubilis L.) by Soft Capsules Composed of Gelatin and Cactus Mucilage Biopolymers. Polymers, 12(9), 1995. https://doi.org/10.3390/polym12091995
Pino, M. Y., Mandujano, N. C., Yépez, A. M., & Abram, A. P. De. (2013). Comparación De Tres Métodos Para Determinar El Porcentaje De Taninos Con El Método De La Norma Astm D6401 Aplicado Para La “Tara”, “Quinual”, “Mimosa” Y “Pino.” Revista de La Sociedad Química Del Perú, 79(4), 381–387.
Rodea-González, D. A., Cruz-Olivares, J., Román-Guerrero, A., Rodríguez-Huezo, M. E., Vernon-Carter, E. J., & Pérez-Alonso, C. (2012). Spray-dried encapsulation of chia essential oil (Salvia hispanica L.) in whey protein concentrate-polysaccharide matrices. Journal of Food Engineering, 111(1), 102–109. https://doi.org/10.1016/j.jfoodeng.2012.01.020
Santana, G. S., Oliveira Filho, J. G. de, & Egea, M. B. (2017). Características tecnológicas de farinhas vegetais comerciais. Revista De Agricultura Neotropical, 4(2), 88–95. https://doi.org/10.32404/rean.v4i2.1549
Santos Teixeira, C. (2009). Farinha da semente se jaca: caracterização físico-química e propriedades funcionais. Universidade Estadual do Sudoeste da Bahia.
Saout, C., Quéré, C., Donval, A., Paulet, Y. M., & Samain, J. F. (1999). An experimental study of the combined effects of temperature and photoperiod on reproductive physiology of Pecten maximus from the bay of brest (France). Aquaculture, 172(3–4), 301–314. https://doi.org/10.1016/S0044-8486(98)00406-2
Shao, Y., & Tang, C. H. (2014). Characteristics and oxidative stability of soy protein-stabilized oil-in-water emulsions: Influence of ionic strength and heat pretreatment. Food Hydrocolloids, 37, 149–158. https://doi.org/10.1016/j.foodhyd.2013.10.030
Silva, K. F. C. e., da Silva Carvalho, A. G., Rabelo, R. S., & Hubinger, M. D. (2019). Sacha inchi oil encapsulation: Emulsion and alginate beads characterization. Food and Bioproducts Processing, 116, 118–129. https://doi.org/10.1016/j.fbp.2019.05.001
Silva, S., Crisóstomo, O., Alvarez, E., Mendoza, G., Rondán, La., & Rubio, J. (2015). Evaluación de propiedades provee la harina de pajuro (Erythrina edulis) a las redes estructurales de Muffins. Revista de Ciencia, Tecnología y Desarrollo, 1(1), 77–88. http://revistascientificas.upeu.edu.pe/index.php/ri_ctd/article/view/360/368
Stone, A. K., Avarmenko, N. A., Warkentin, T. D., & Nickerson, M. T. (2015). Functional properties of protein isolates from different pea cultivars. Food Science and Biotechnology, 24(3), 827–833. https://doi.org/10.1007/s10068-015-0107-y
Stone, A. K., Nosworthy, M. G., Chiremba, C., House, J. D., & Nickerson, M. T. (2019). A comparative study of the functionality and protein quality of a variety of legume and cereal flours. Cereal Chemistry, 96(6), 1159–1169. https://doi.org/10.1002/cche.10226
Sun, L. H., Lv, S. W., Chen, C. H., & Wang, C. (2019). Preparation and characterization of rice bran protein-stabilized emulsion by using ultrasound homogenization. Cereal Chemistry, 96(3), 478–486. https://doi.org/10.1002/cche.10147
Taha, A., Hu, T., Zhang, Z., Bakry, A. M., Khalifa, I., Pan, S., & Hu, H. (2018). Effect of different oils and ultrasound emulsification conditions on the physicochemical properties of emulsions stabilized by soy protein isolate. Ultrasonics Sonochemistry, 49, 283–293. https://doi.org/10.1016/J.ULTSONCH.2018.08.020
Vélez-Erazo, E. M., Bosqui, K., Rabelo, R. S., Kurozawa, L. E., & Hubinger, M. D. (2020). High internal phase emulsions (HIPE) using pea protein and different polysaccharides as stabilizers. Food Hydrocolloids, 105, 105775. https://doi.org/10.1016/j.foodhyd.2020.105775
Vélez-Erazo, E. M., Consoli, L., & Hubinger, M. D. (2018). Mono and double-layer emulsions of chia oil produced with ultrasound mediation. Food and Bioproducts Processing, 112, 108–118. https://doi.org/10.1016/j.fbp.2018.09.007
Vicente, J., Pereira, L. J. B., Bastos, L. P. H., de Carvalho, M. G., & Garcia-Rojas, E. E. (2018). Effect of xanthan gum or pectin addition on Sacha Inchi oil-in-water emulsions stabilized by ovalbumin or tween 80: Droplet size distribution, rheological behavior and stability. International Journal of Biological Macromolecules, 120, 339–345. https://doi.org/10.1016/j.ijbiomac.2018.08.041
Vidaurre-Ruiz, J., Matheus-Diaz, S., Salas-Valerio, F., Barraza-Jauregui, G., Schoenlechner, R., & Repo-Carrasco-Valencia, R. (2019). Influence of tara gum and xanthan gum on rheological and textural properties of starch-based gluten-free dough and bread. European Food Research and Technology, 245(7), 1347–1355. https://doi.org/10.1007/s00217-019-03253-9
Zhang, J., Wang, G., Liang, Q., Cai, W., & Zhang, Q. (2019). Rheological and microstructural properties of gelatin B/tara gum hydrogels: Effect of protein/polysaccharide ratio, pH and salt addition. LWT, 103, 108–115. https://doi.org/10.1016/J.LWT.2018.12.080
Zhou, L., Zhang, J., Xing, L., & Zhang, W. (2021). Applications and effects of ultrasound assisted emulsification in the production of food emulsions: A review. Trends in Food Science and Technology, 110(1), 493–512. https://doi.org/10.1016/j.tifs.2021.02.008
[1]
[3]
[4]