Artículo Original
MORPHOMETRIC DISTINCTION OF DOMESTIC AND SYLVATIC POPULATIONS OF Rhodnius ecuadoriensis FROM DIFFERENT GEOGRAPHICAL ORIGINS
DISTINCIÓN MORFOMÉTRICA DE POBLACIONES DOMÉSTICAS Y SILVESTRES DE Rhodnius ecuadoriensis DE DIFERENTES ORIGENES GEOGRÁFICOS
JS. Patterson+ F. Abad- Franch +, CA. Cuba Cuba++ & MA. Miles+
+ Pathogen Molecular Biology and Biochemistry Unit. Department of Infectious and Tropical Diseases.
London School of Hygiene and Tropical Medicine. London – England.
++ Unidade de Parasitologia Médica e Biologia de Vetores. Faculdade de Medicina - Universidade de Brasília, Brasil.
Autor para correspondencia: cesaracuba@gmail.com
Recibido: 5 de abril, 2020. Aceptado: 19 de mayo, 2020
ABSTRACT
Rhodnius ecuadoriensis (Hemiptera, Reduviidae) is an important vector of both Trypanosoma hemoflagellates, Trypanosoma cruzi and Trypanosoma rangeli, in Ecuador and Peru. Ecotopes of sylvatic and domestic/peridomestic habitats have been reported in Ecuador. Meanwhile in Peru, to the best of our knowledge, findings of sylvatic populations in their different ecosystem regions have not yet been documented. Could this be the product of a lack of appropriate studies on wild populations of triatominae in Peruvian environments? In order to elucidate this topic, we take advantage of new insights in geometric morphometry as a tool to help differentiate between wild populations and the corresponding domestic/peridomestic ones, collected in their respective environments. When analyzing our results, we confirmed the efficacy of this technique in our study, and furthermore, we believe that it could be a proper tool for rangeliosis and Chagas disease vector control surveillance in Ecuador and Peru.
Keywords: Morphometric geometry, sylvatic, domestic/peridomiestic populations distinction, Rhodnius ecuadoriensis.
RESUMEN
Rhodnius ecuadoriensis (Hemiptera, Reduviidae) es un importante vector de los tripanosomas Trypanosoma cruzi y Trypanosoma rangeli en el Ecuador y Perú. Se han reportado ecotopos de hábitats silvestres y domésticos/peridomésticos en Ecuador. Sin embargo, en Perú, hasta donde sabemos, no se ha documentado hallazgos de dichas poblaciones silvestres. ¿Podría este ser el caso de una falta de estudios focalizados en la búsqueda de poblaciones silvestres de triatominos dentro de los diferentes ecosistemas del Perú? Para elucidar este tema, aplicamos nuevas perspectivas en morfometría geométrica, como una herramienta que podría auxiliar en la diferenciación de poblaciones silvestres de aquellas domésticas/peridomésticas, colectadas en sus respectivos ambientes naturales. Al analizar nuestros resultados, se confirmó la utilidad de esta técnica dentro de nuestro estudio, y esto nos llevó a creer asimismo que serviría como un elemento apropiado en el control vectorial de la enfermedad de Chagas y de la rangeliosis, en Ecuador y Perú.
Palabras claves: Geometría morfométrica, silvestre, diferenciación de poblaciones domesticas/peridomesticas, Rhodnius ecuadoriensis.
DOI: http://dx.doi.org/10.17268/rebiol.2020.40.01.01
1. INTRODUCTION
Rhodnius ecuadoriensis (Lent y Leon, 1958) is a significant vector of Chagas disease in the Western side of the Andes of Central - Southern Ecuador. In Peru, this insect is the vector of Trypanosoma rangeli, even though its role on the epidemiology of Trypanosomiasis americana has not yet been defined (Cuba Cuba et al., 2002). Sylvatic populations breed in Phytelephas aequatorialis palm trees in subtropical valleys of Pichincha, Ecuador (Abad Franch et al., 2001). R. ecuadoriensis has a natural preference for a dry and xerophytic ecosystem, which is commonly seen in Northern Perú where no palms species are found. The species seems to be exclusively associated with human environments in southern Ecuador and over its entire range in Peru. In a preliminary work, some phenetic differences were recorded between sylvatic and domestic/peridomestic specimens (Abad-Franch, 2000; Patterson et al., 2002). Here we characterize those differences using multivariate statistics of morphometric variable, a technique that is able to discriminate among conspecific bug populations of sylvatic and domestic origin (Dujardin et al., 1997).
2. MATERIAL AND METHODS
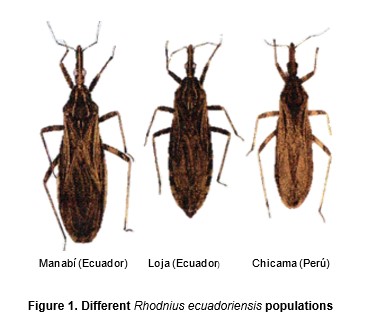
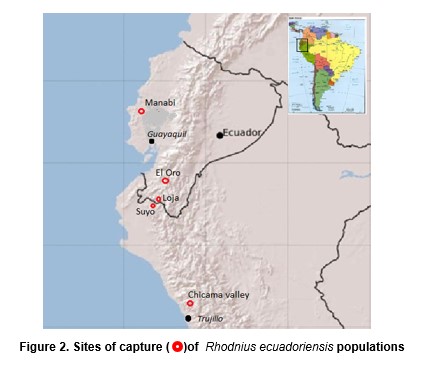
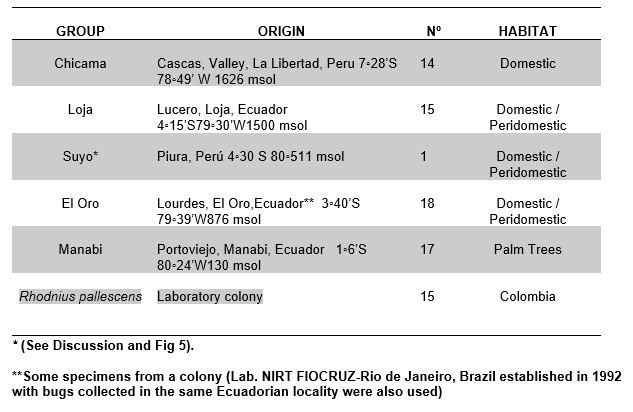
A total of 65 adult R. ecuadoriensis (classified after Lent y Wygodzinsky, 1979) from four populations were studied: Chicama, Loja/Suyo, El Oro and Manabi (Table 1, and Figures 1 and 2). Rhodnius pallescens (15 individuals) was used an outgroup (here called pallescens). Bugs from Peru were collected by Cuba (Cuba et al., 2003), in the Cascas valley, Chicama (Figures 2A, 2B).
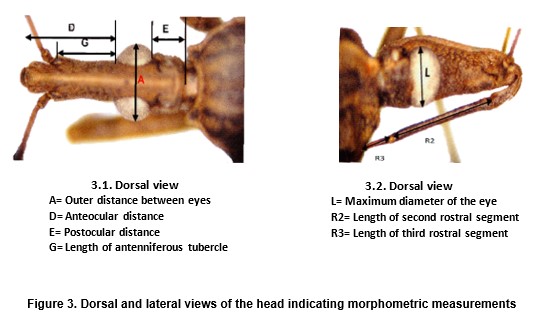
Seven parameters were considered when measuring the head of each bug (Fig 3, 1 and 3, 2). Data was obtained by using a digital video camera mounted on a light dissection microscope and analysed by isometry-free canonical variate analysis (CVA). Log- transformed datasets were centred by row and submitted to principal component analysis, the first six principal components (PC) were used as input for canonical variate analysis. The first two canonical factors (CV 1 and CV 2) were subsequently used to construct a factorial map. Mahalanobis distances were submitted to an Unweighted Pair Group Method with Arithmetic mean (UPGMA) cluster analysis. A dendrogram was constructed showing the relationships between populations. The JMP and STATA 7 software packages were utilized. Size differences were analyzed by ANOVA, using the mean values of all log -transformed measurements of each bug (“isometric estimator of size”).
3. RESULTS
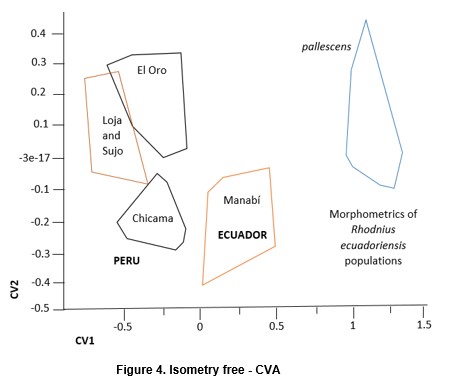
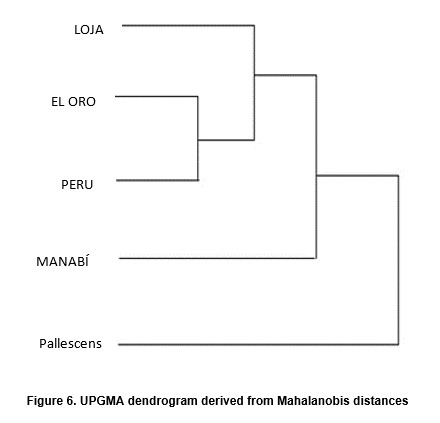
Factorial maps derived from CVA (Figures 4 and 5) detected important differences between the sylvatic population of R. ecuadoriensis and those of peridomestic and/or domestic origins. There was also little overlap between domestic/peridomestic bugs from Ecuador and domestic Peruvian specimens from Cascas valley, Chicama (Table 1). However, these three groups showed limited variability among them. The UPGMA dendrogram (Figure 6) also reflects the separation between domestic ad sylvatic R. ecuadoriensis populations.
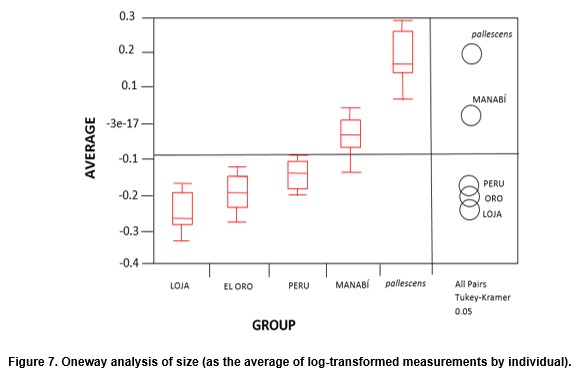
The fact that the Chicama group is clustered together with El Oro is probably related to similarities in size (see Figure 7). In fact, the contribution of size (allometric residuals) to CV was important when examined by regression analysis. No differences were recorded between field-collected bugs from Manabi and those from the laboratory colony. Bugs of sylvatic origin were significantly bigger (p < 0.05) than synanthropic ones. (Figure 7). In addition, bugs from Loja-Suyo were smaller than those from Chicama and El Oro, which were very similar in size.
Table 1. Populations used in this study
4. CONCLUSIONS AND DISCUSSION
1. Utility of morphometric variables
Isometry-free morphometric analysis can establish clear-cut differences between sylvatic and synanthropic populations of R. ecuadoriensis. Thus, metric variables could be used to monitor the origin of re-infestations after residual spraying and it may be give clues to explain the domiciliation process of the species. As pointed out, sylvatic triatominae constitute a challenge in vector control transmission (Guhl et al., 2009), so viable control strategies must emphasize the presence of bugs populations in wild ecotopes.
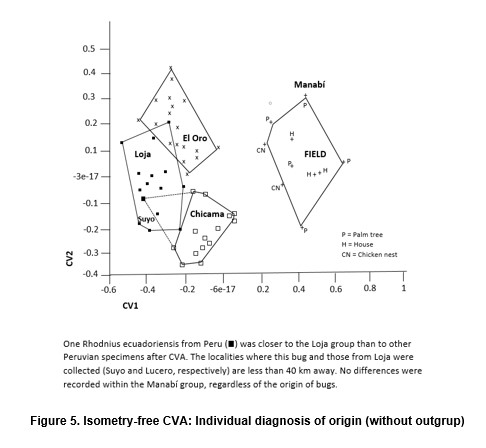
Differences between the Manabi population (sylvatic) and the El Oro-Loja Peru cluster (domestic) were easily detected using this approach. The sensitivity of the morphometric analysis was further demonstrated when a single bug distorted the Cascas, Chicama cluster (Figure 5). When the origin of bugs was checked, it was discovered that this single specimen had actually been collected from Suyo, a Peruvian locality a few km away from the site of capture of the Loja specimen (Lucero, see Figure 2), to which cluster it was assignated by the morphometric analysis. That group was therefore renamed (Loja-Suyo) and the Suyo specimen included in it. However, the largest part of the intraspecific variation detected does not seem to be geographic in nature. The Cascas, Chicama group (Table 1) bugs are most similar to those of El Oro, a distant locality. In general, all the synanthropic populations are similar to each other, and all of them are clearly different from the sylvatic one. These differences are therefore likely to reflect the distinct habitat.
Our results suggest that a baseline set of metric variables including different local populations could be used to investigate the origin of any re-infesting bug (see also Schofield, 2001). Therefore, the use of geometric morphometry could help overcome the influence of size in the analysis (Jaramillo, 2000).
2. Possible isolation of populations
The strong separation between Manabi and the rest of populations might also reflect a certain degree of genetic isolation. In fact, the palm trees that constitute the primary natural habitat do not exist in the arid north of Peru, nor in the Valley of Loja. Only in El Oro, scattered remnants of humid forests with Phytelephas palms can be found (cf. Abad-Franch et al, 2001). Together with the present results, this suggests that domestic and peridomestic populations of southern Ecuador and northern Peru (i.e. the epidemiological significant ones) are probably isolated from sylvatic foci associated with Phytelephas palms. Consequently, local eradication of those synanthropic populations may be attainable through residual insecticide spraying. Population genetic studies based on molecular approaches will contribute to clarify the relationships between these populations.
5. ACNOWLEDGEMENTS
Supported by the ECLAT network. UNDP/ World Bank TDR (grant 970195), CAPES Ministerio de Educação e Cultura, Brasil), the Oficina Nacional de Epidemiología (Instituto Nacional de Salud. Ministerio de Salud Pública, Perú), the University General Hospital of Valencia, Spain and the Cañada-Blanch Foundation, Spain. F. Guhl and J. Jurberg kindly provided colony specimens from their laboratories.
6. REFERENCES
Abad-Franch, F. 2000. Ecology and genetics of Chagas disease vectors in Ecuador. Implications for the design of control strategies. MPhil-Phd Upgrading Report. London School of Hygiene and Tropical Medicine. University of London, UK. 150 pp.
Abad-Franch, F., Paucar, C., Carpio, C., Cuba Cuba C., Aguilar, V. y Miles, M. 2001. Biogeography of Triatominae (Hemiptera: Reduviidae) in Ecuador: Implications for the design of control strategies. Memorias do Instituto Oswaldo Cruz, 96. 611-620.
Cuba Cuba CA., Abad-Franch, F., Roldan, J., Vargas, F., Pollack, L. y Miles, M. 2002. The Triatomines of Northern Peru, with emphasis on the Ecology and infection by Trypanosomes of Rhodnius ecuadoriensis (Triatominae). Memorias do Instituto Oswaldo Cruz 97, 175-183.
Cuba Cuba C., Vargas, F., Roldan, J. y Ampuero, C. 2003. Domestic Rhodnius ecuadoriensis (Hemiptera: Reduviidae) infestation in Northern Peru A comparative trial of detections methods during a six-month follow-up. Revista do Instituto de Medicina Tropical de São Paulo. São Paulo 45 (85-9092).
Dujardin, J., Bermudez, H., Casini, C., Schofield, C. y Tibayrenc, M. 1997. Metric differences between sylvatic and domestic Triatoma infestans (Hemiptera: Reduviidae) in Bolivia. Journal of Medical Entomology.34, 544-551.
Guhl, F., Pinto, N. y Aguilera, G. 2009. Sylvatic triatominae a new challenge in vector control transmission. Memorias do Instituto Oswaldo Cruz 104 (Suppl. 1): 71-75.
Jaramillo, N. 2000. Partición en tamaño y forma de los caracteres métricos y su interés en los estudios poblacionales aplicados a los Triatominos. PhD Thesis, University of Antioquia, Medellin, Colombia, 125 pp.
Lent, H. y León, L. 1958. Um novo Rhodnius Stäl do Ecuador (Hemiptera, Reduviidae). Rev Brasil Biol 18: 181-185.
Lent, H. y Wygodzinsky, P. 1979. Revision of the Triatominae (Hemiptera: Reduviidae), and their significance as vectors of Chagas disease. Bulletin of the American Museum of Natural History, 163(3), 123-520.
Patterson, J., Abad-Franch, F., Aguilar, V., Cuba Cuba, C. y Miles, M. 2002. Morphometrics of Rhodnius ecuadoriensis (Triatominae) populations: a tool for Chagas disease vector control surveillance in Ecuador and Perú. Transactions of the Royal Society of Tropical Medicine and Hygiene. 96 (4)353-464.
Schofield, C. 2001. Field testing and evaluation of insecticides for indoor spraying against domestic vectors of Chagas disease. WHO/CDC/WHOPES/GCDPP/2001. 1WHO, Geneva, Switzerland, 53 pp.
FIGURES