Isolation of wild fermentative yeast strains from native fruits of Misiones province, Argentina
Aislamiento de cepas de levadura fermentativa silvestre a partir de frutas autóctonas de la provincia de Misiones, Argentina
Ana P. Butiuk*; Silvana A. Maidana; Aldana E. Godoy; María A. Martos; Emilce R. Zubreski
School of Exact, Chemical and Life Sciences, Misiones National University, Félix de Azara 1552, N3300LQH Posadas, Misiones, Argentina.
ORCID de los autores:
A. P. Butiuk: https://orcid.org/0000-0003-4043-7752 S. A. Maidana: https://orcid.org/0000-0002-3115-9538
A. E. Godoy: https://orcid.org/0009-0007-4011-3017 M. A. Martos: https://orcid.org/0000-0003-1235-0529
E. R. Zubreski: https://orcid.org/0009-0004-2306-5285
ABSTRACT
The objective of the present study was to isolate and select wild fermentative yeast strains to be used in the production of regional alcohol beverages. Yeasts were isolated from the spontaneous fermentation of must from different fruits of Misiones province and their ability to produce ethanol, as well as their tolerance to stress factors, were evaluated. Among a total of 62 isolated yeast strains, P2, P6, P7, and P11 (isolated from pitanga), and M31 (isolated from black mulberry) were preselected for presenting greater fermentation capacity. Strains P6 and P11 proved to be the most resistant to ethanol and more osmotolerant, could grow in the presence of SO2 and presented a good ethanol yield, which was 69.6% (P6) and 77% (P11) with respect to the maximum theoretical yield. The other yeast strains showed lower tolerance to the stress factors studied. According to the results of the present study, strains P6 and P11 were selected for further research focused on the production of regional fruit wines.
Keywords: wild yeasts; fruits; fermentation; osmotolerance; ethanol tolerance.
RESUMEN
El objetivo de este estudio fue aislar y seleccionar cepas de levaduras fermentativas silvestres para la producción de bebidas alcohólicas regionales. Las levaduras se aislaron a partir de la fermentación espontánea del mosto de diversas frutas de la provincia de Misiones, y se evaluó su capacidad para producir etanol, así como su tolerancia a factores de estrés. De un total de 62 cepas aisladas, las cepas P2, P6, P7 y P11 (aisladas de pitanga) y M31 (aislada de mora negra) fueron preseleccionadas por presentar mayor capacidad fermentativa. Las cepas P6 y P11 demostraron mayor resistencia al etanol y mayor tolerancia osmótica, además de poder crecer en presencia de SO₂ y presentar un buen rendimiento de etanol: 69,6% (P6) y 77% (P11) respecto al rendimiento teórico máximo. Las demás cepas mostraron menor tolerancia a los factores de estrés estudiados. En base a estos resultados, las cepas P6 y P11 fueron seleccionadas para futuras investigaciones enfocadas en la producción de vinos de frutas regionales.
Keywords: levaduras silvestres; frutas; fermentación; tolerancia osmótica; tolerancia al etanol.
1. Introduction
Alcoholic fermentation is the anaerobic transfor-mation of sugars into ethanol and carbon dioxide by the action of yeast. All fruits, from the most common to the most exotic tropical fruits, can be used to produce alcoholic beverages through the fermentation process. These alcoholic beverages retain both the flavor and most of the nutrients present in fruits and their nutritional value increa-ses thanks to the release of amino acids and other nutrients due to the metabolism of yeast during the fermentation process.
Fermented alcoholic beverages derived from fruits other than grapes are generally called "fruit wines." The process of making fruit wines is similar to that of grape wine. Fruit wines contain between 8% to 11% (v/v) alcohol and 2% to 3% (w/v) sugar (Voget et al., 2013; Ajit et al., 2018; Haro-Altamirano et al., 2021).
Yeasts play a fundamental role in the production of fermented beverages because, in addition to being responsible for alcoholic fermentation, they generate other secondary compounds as products of their metabolism (organic acids, aldehydes, esters, higher alcohols, sulfur compounds, etc.) which influence the aromas and flavors and contribute to the organoleptic profile of the final product (Suárez-Valles et al., 2005; Lorenzini et al., 2019).
The use of pure cultures of native yeast isolated from natural sources in a given region is of great interest for the production of fruit wines, since these strains are adapted to both the local climatic conditions and the natural source (fruit) from which they were isolated, and therefore will transmit unique organoleptic characteristics to the fermented beverage (Ajit et al., 2018; Santamaría et al., 2008).
During fermentation, yeasts are exposed to stress conditions such as those produced by high initial sugar concentrations and high concentrations of ethanol produced, which considerably affect growth and fermentation efficiency. Yeast strains selected for use in alcoholic fermentation industries must present specific characteristics, such as, tolerance to high sugars and ethanol concentrations, tolerance to pH variations, production of aromatic compounds, genetic stability, etc. (Mercado et al., 2007). During the winemaking process, SO2 is added as an antioxidant and antiseptic in a procedure known as sulfite treatment, so the yeasts selected to carry out alcoholic fermentation must tolerate an SO2 concentration of 60 mg/L or higher, frequently used in winemaking (Voget et al., 2013; Miranda-Castilleja et al., 2015).
Fruit wines represent an attractive alternative to promote agro-industrial development associated with the valorization of regional fruits to produce beverages with novel sensory properties (Voget et al., 2013; Loyo-Trujillo et al., 2020). The Province of Misiones, due to its climatic conditions, has an innumerable variety of plant species and a great variety of fruits that can be used for the production of alcoholic beverages.
The objective of the present study was to isolate and select new yeast strains with fermentative potential from the spontaneous fermentation of musts from native fruits of the province of Misiones, Argentina, to be used as starter cultures in the production of alcoholic beverages, in order to obtain a product with unique sensory characteristics, typical of this region.
2. Methodology
2.1 Natural sources for yeast isolation
Yeasts were isolated from fruits collected from the Native Species Nursery belonging to the Ministry of Ecology of Misiones Province (Garupá, Misiones). Fruits with no evidence of micro-organisms or insects were collected, transported to the laboratory under refrigeration, and stored at 5 °C before use and for no more than 48 h.
2.2 Culture media
Enrichment broth (g/L): yeast extract (Sigma), 5; tryptone (Difco - Becton Dickinson), 5; bacteriolo-gical glucose (Britania), 10; calcium propionate, 1.3; oxytetracycline (Pfizer), 100 mg/L; pH 4.5. The culture medium was sterilized for 15 min at 121 °C, except for the oxytetracycline solution, which was sterilized separately by filtration through 0.22 μm pore size cellulose nitrate membranes (Sartorius AG, Goettingen, Germany). The solutions were mixed aseptically before use.
Isolation medium: same composition as the enrichment broth, but with the addition of agar, 15 g/L. As mentioned above, the medium and antibiotic solution were sterilized separately. The sterile medium was cooled to 45 °C, the antibiotic solution was added, homogenized, and immediately distributed into Petri dishes.
Conservation medium (g/L): yeast extract, 5; tryptone, 5; glucose, 15; agar (Britania), 15; pH: 4.5. The culture medium was sterilized at 1 atm for 15 min.
Fermentation medium (g/L) (semi-synthetic): gluco-se, 20; (NH4)2SO4, 1.5; yeast extract, 1; MgSO4. 7H2O, 0.5; K2HPO4, 3; KH2PO4, 2; pH 4.5. The glucose, phosphate, and other components were sterilized separately for 15 minutes at 121 °C. The corresponding amounts of each solution were aseptically mixed before use (Maidana et al., 2019).
Basal medium (g/L): KH2PO4, 1; MgSO4.7H2O, 0.5; peptone, 8; yeast extract, 4, pH: 4.5. The phosphate and the rest of the components were sterilized separately us it was mentioned previously (Peña & Arango, 2009).
2.3 Wild yeast isolation
2.3.1 Must preparation
The fruits were washed, properly separated from their seeds according to the type of fruit, and ground with a food processor for 1 minute. After adjusting the pH, the resulting must (juice and pulp) was distributed by adding 50 mL to 250 mL Erlenmeyer flasks containing 30 mL of enrichment broth and stored at 5 °C for no more than 24 h.
2.3.2 Spontaneous fermentation and yeasts isolation
The Erlenmeyer flasks containing must and enrichment broth were left to ferment spontaneously at 30 °C, for 8 days. For yeast isolation, 0.5 mL of the fermented broth was inoculated into tubes containing 10 mL of enrichment broth. The tubes were incubated at 30°C for 24 h. Then, an aliquot of each culture was serially diluted, plated in isolation medium and incubated at 30°C for 24-48 h. To obtain pure cultures, well-separated colonies that showed typical yeast morphology were suspended in physiological saline solution and cultured in Petri dishes again. The yeasts isolated were maintained on conservation medium agar slants, at 5°C (Martos et al., 2013).
2.4 Selection of fermentative yeasts (screening)
The ability of the isolated yeasts to produce ethanol in the fermentative medium (semi-synthetic medium) was evaluated. Five hundred milliliter Erlenmeyer flasks with 280 mL of fermentation medium were inoculated with 20 mL of an appropriate dilution of a suspension of the microorganism (Abs620 = 0,96), grown in conservation medium (30 °C, 24 h). The Erlenmeyer flasks were equipped with an airlock valve to ensure anaerobic growth and incubated at 30 °C for 5 days, in a thermostatic bath. The biomass was separated by centrifugation (2350×g, at 5 C, for 10 min). The supernatant was distilled and the alcoholic content of the distillate was determined.
2.5 Sugar tolerance of isolated yeasts
Osmotolerance tests were performed in basal medium supplemented with different concen-trations of glucose (20, 50, 100, 150, 200 or 250 g/L). The ability of the selected yeast strains to grow in the presence of sucrose (50 g/L) as sole carbon and energy source (CES) was also evaluated. Cultures were carried out in Erlenmeyer flasks containing growth medium with the corresponding initial glucose concentration. The Erlenmeyer flasks were inoculated with an appropriate dilution of a suspension of the microorganism (Abs620 = 0,96), grown in conservation medium (30 °C, 24 h) and incubated at 30 °C, for 48 h, in a rotary shaker incubator (MRC, TOU-50N, 25 mm shaking width) at 150 rpm. A commercial yeast (S. cerevisiae) was used as reference strain (Miranda-Castilleja et al., 2015; Canché-Collí et al., 2021).
2.6 Ethanol tolerance of isolated yeasts
The ethanol tolerance analysis of the wild yeast strains was tested in a basal medium containing 20 g/L of glucose and supplemented with increasing concentrations of ethanol (from 4 to 12% v/v). Yeast cultures were performed as previously described for sugar tests. A control culture was grown for each strain, to which no ethanol was added. S. cerevisiae was used as reference strain (Miranda-Castilleja et al., 2015; Ortiz-Zamora et al., 2009).
2.7 Sulfur dioxide tolerance of isolated yeasts
The sulfur dioxide (SO2) tolerance of the wild yeast strains was tested in a basal medium containing 20 g/L of glucose and supplemented with a potassium metabisulfite (MBK) solution (10%, v/v) to obtain 65 and 130 mg/L of SO2 in the culture medium (Voget et al., 2013; Miranda-Castilleja et al., 2015). Yeast cultures were performed as previously described for sugar tests. A control culture was grown for each strain, to which no K2O5S2 solution was added. S. cerevisiae was used as reference strain.
2.8 Alcoholic fermentation
Alcoholic fermentations were conducted in the semi-synthetic medium containing 100 g/L glucose as previously described (section 4). The ethanol yield (Yp/s, w/w) of the selected strains was calculated as the ethanol produced per sugar consumed. The theoretical ethanol yield according to Gay-Lussac is 0.51 gethanol/gglucose.
2.9 Analytical techniques
Biomass: growth was followed by measuring the optical density of yeast suspensions at 620 nm.
Glucose: glucose was determined using the glucose oxidase-peroxidase (Glicemia, Wiener, Argentina) method.
Ethanol: the alcoholic content of the distillate was determined with a calibrated alcoholmeter with a tolerance of ± 0.1% v/v and expressed as a percentage of ethanol (%, v/v). The temperature was corrected by table.
2.10 Statistical analysis
Determinations were carried out by triplicate and data were expressed as means ± standard deviation. The results were analyzed by simple ANOVA using a Statgraphics Centurion XV statistical program.
3. Results and discussion
3.1 Natural sources for yeast isolation
The fruits found in the Native Species Nursery of the Ministry of Ecology (Misiones Province) that were used to isolate the yeasts with their respective scientific names, are presented in Table 1 and Figure 1. They have been selected based on their characteristics and availability in the area.
Black cerella (Eugenia involucrata) is a tree with white flowers belinging to Myrtaceae family that naturally grows in Misiones and Corrientes provinces (Argentina). Its fruits are fleshy, almost spherical (about 2 cm in diameter), purple and contain 1 or 2 seeds inside (Figure 1A). Fruit ripening occurs between September and October (Girardeloet al., 2020). Passion fruit (Passiflora edulis) whose flower is called passionflower, is a species of climbing plant of the Passifloraceae family, that grows wild in several South American countries. The fruit is an orange-colored berry, round or oval in shape with yellow spots. Its pulp is juicy and gelatinous, while the seeds are small, hard and brown (Figure 1B) (Campos-Rodriguez et al., 2023). Black mulberry (Morus nigra), of Persian origin, was introduced to Misiones and adapted very well to the climate. Mulberry is the name given to various edible fruits of different species; whose fruit is an eterio, composed of small drupes (between 1 and 3 cm). The color varies as the mulberry matures depending on the species. Unripe Morus nigra has a greenish-white tone, then turns red, and at the end of ripening is black (Figure 1C) (Gundogdu et al., 2011; Koyuncu et al., 2014). Pindó (Syagrus romanzoffiana) belongs to the Arecaceae family like all palm trees. It is native to Brazil, Paraguay, Uruguay, and Argentina. In Argentina, it is present in several northeastern provinces, including Misiones. The pindó fruit has a drupaceous, globose-ovoid structure, orange-yellow in color that resembles a date (25 mm long by 15 mm in diameter). It contains a single seed, ovoid and pointed at both ends. The pulp, though not abundant, is sweet, fibrous, and somewhat gummy, edible, and has a pleasant taste (Figure 1D) (Rompato et al., 2015) Pitanga (Eugenia uniflora) is a small tree of the Myrtaceae family, native to South America. It blooms in spring, and again in mid-summer. The fruit is an oblate berry (up to 4 cm in diameter) with eight visible ribs, which turns from green to orange and dark purple as it ripens. The pulp of the fruit is red, juicy, sweet to sub-acid depending on the degree of ripeness, with one spherical seed or two or three flattened ones (Figure 1E) (Chacón-Ordóñez et al., 2013).
Table 1
Native fruits of Misiones province
Common name | Scientific name | Family |
Black cerella | Eugenia involucrata | Myrtaceae |
Passion fruit | Passiflora edulis | Passifloraceae |
Black mulberry | Morus nigra | Moraceae |
Pindó fruit | Syagrus romanzoffiana | Arecaceae |
Pitanga | Eugenia uniflora | Myrtaceae |
Jabuticaba | Plinia cauliflora | Myrtaceae |
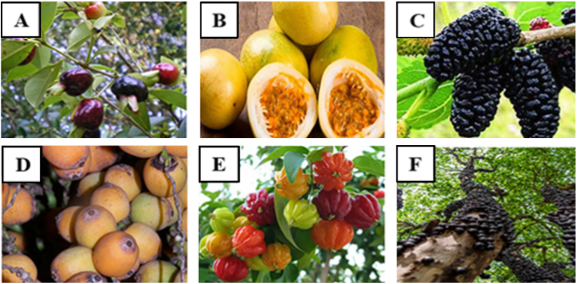
Figure 1. Black cerella (A), passion fruit (B); black mulberry (C), pindó fruit (D), pitanga (E), jabuticaba (F).
Jabuticaba (Plinia cauliflora) is a tree belonging to the Myrtaceae family, native to Brazil, Argentina, Bolivia, and Paraguay. Its fruits give the impression of being attached to the stem. They are purple at first and black when ripe. The fruit is classified as a berry and is highly perishable. It has a thin and smooth skin, while its pulp is white and juicy with a sweet and sour taste (Figure 1F) (Alezandro et al., 2013).
All these fruits are rich in polyphenols, that give their importance as an antioxidant, sugars, vita-mins (A, C, E and B-complex vitamins), minerals (Ca, P, K, Mg, Fe, Zn, Na), fibers and carotenoids) (Girardelo et al., 2020; Campos-Rodriguez et al., 2023; Gundogdu et al., 2011; Koyuncu et al., 2014; Rompato et al., 2015; Chacón-Ordóñez et al., 2013; Alezandro et al., 2013).
3.2 Wild yeast isolation
Table 2 shows the number of yeast strains isolated and the nomenclature assigned to each of them.
Table 2
Yeasts isolated from spontaneous fermentations of fruit musts
Source | No. of strains | Nomenclature |
Pitanga | 14 | P2 - P15 |
Black mulberry | 20 | M16 - M36 |
Black cerella | 8 | C37 - C45 |
Jabuticaba | 2 | J46 - J47 |
Pindó | 12 | Pin48 - Pin60 |
Passion fruit | 2 | Pa61- Pa62 |
A total of 62 strains with typical morphological characteristics of yeasts (colonies with creamy texture, smooth and shiny surface, lobed edges and rounded or oval individual cells) were isolated from the spontaneous fermentation of musts from different native fruits of Misiones province (Guevara-Bravo et al., 2014).
3.3 Selection of fermentative yeasts (screening)
Table 3 shows the percentage of alcohol produced by the different isolated yeast strains. All yeasts (62 strains) grew in the semi-synthetic culture medium, of which 16 strains (26%) were able to produce ethanol: 7 from pitanga (P2, P6, P7, P9, P11, P12 and P15); 5 from black mulberry (M16, M17, M23, M27 and M31), 2 from cerella (C40, C44), 1 from jabuticaba (J46), and 1 from pindó (Pin48). The other strains were non-fermentative. Five strains (P2, P6, P7, P11 and M31) were able to produce ~1% (v/v) of ethanol. These yeast strains were selected for further studies considering that the others strains yielded a lower ethanol percentage (< 1%, v/v).
Table 3
Alcohol produced by the yeasts isolated from the different native fruits
Fruit | Strain | Alcohol (% v/v) |
Pitanga | P3-P5; P8; P10; P13-P14 | nd |
P9; P12; P15 | ˂ 1 |
P2; P6-P7; P11 | ~1 |
Black mulberry | M18-22; M24-26; M31 | nd |
M16; M17; M23; M27 | ˂ 1 |
M28-M30 | ~1 |
Black cerella | C37-C39; C41-43 | nd |
C40; C44 | ˂ 1 |
Jabuticaba | J47 | nd |
J46 | ˂ 1 |
Pindó | Pin49-60 | nd |
Pin48 | ˂ 1 |
Passion fruit | Pa61-62 | nd |
nd: not detected.
3.4 Sugar tolerance of isolated yeasts
The effect of increasing the initial glucose concen-tration on growth of the selected yeast strains is shown in Table 4. Analysis of variance determined that sugar concentration significantly influenced the growth of yeast strains (p ≤ 0.05). As shown in Table 4, high sugar concentrations inhibit yeasts growth. The growth of the yeast strain P2 decreased from 150 g/L of glucose. P6, P11 and M31 strains were the most osmotolerant and grew with initial sugar concentrations of 200 g/L; at higher glucose concentrations a slight decrease in growth was observed.
Table 4
Growth of the isolated yeasts with different initial glucose concentrations
Glucose (g/L) | ΔAbs620 |
Strain P2 | Strain P6 | Strain P7 | Strain P11 | Strain M31 | Control |
20 | 0.66 ± 0.014 | 0.80 ± 0.005 | 0.65 ± 0.020 | 0.57 ± 0.008 | 0.63 ± 0.017 | 0.75 ± 0.002 |
50 | 0.80 ± 0.025 | 0.98 ± 0.004 | 0.72 ± 0.032 | 0.65 ± 0.004 | 0.77 ± 0.009 | 0.92 ± 0.003 |
100 | 0.87 ± 0.019 | 1.07 ± 0.028 | 0.63 ± 0.006 | 0.81 ± 0.023 | 0.84 ± 0.002 | 0.71 ± 0.128 |
150 | 0.66 ± 0.013 | 1.16 ± 0.023 | 0.52 ± 0.005 | 0.82 ± 0.005 | 0.86 ± 0.021 | 0.50 ± 0.011 |
200 | 0.55 ± 0.007 | 1.05 ± 0.046 | 0.30 ± 0.007 | 0.92 ± 0.004 | 0.95 ± 0.003 | 0.27 ± 0.001 |
250 | 0.47 ± 0.007 | 0.81 ± 0.023 | 0.25 ± 0.002 | 0.80 ± 0.002 | 0.83 ± 0.011 | 0.20 ± 0.002 |
Abs620 mean ± SD. Dilution of the culture: 1:4. ΔAbs620= Absfinal – Absinitial.
P7 strain and commercial yeast were the least tolerant and a decrease in growth was observed from 100 g/L of initial glucose.
High sugar concentrations inhibit growth due to the increase in the osmotic pressure of the medium. Some authors have reported that substrate inhibition of growth occurs in sugar range of 5%-20% (w/v) with complete growth inhibition at 25%–40% (w/v) of glucose, depending on the strain (Canché-Collí et al., 2021; Malacrino et al., 2005). Ortiz Zamora et al. (2009) reported that 4 yeast strains isolated from natural sources in Veracruz (México) (sugarcane juice, sugarcane molasses and grape juice), showed glucose tolerance up to 20% (w/v), which was higher than that of the commercial strain S. cerevisiae K1, used as a reference. Canché-Collí et al. (2021) evaluated the growth of yeast strains isolated from flower nectar (Metschnikowia koreensis and Sympodiomycopsis paphiopedili) and honey of Melipona beecheii (Starmerella apicola and Starmerella apicola 2), in a medium with different glucose concentrations (2%, 10%, 20%, 40% and 60%, w/v). These authors reported that the growth of the yeasts decreased as the glucose concen-tration increased above 20% (w/v), with higher growth observed in yeasts isolated from honey.
Figure 2 shows the growth results of P2, P6, P7, M31 strains and the reference strain in the presence of sucrose (50 g/L) as the sole carbon and energy source (CES).
Figure 2 shows that the yeast strains were able to growth with sucrose as CES. Greater growth was observed with P11 strain, followed by P6 and M31 strains. Due to the relatively low sugar level of fruit juices, it is common practice to add sucrose at the beginning of fermentation to obtain wines with higher alcohol content (Ajit et al., 2018; Loyo-Trujillo et al., 2020; Canché-Collí et al., 2021).
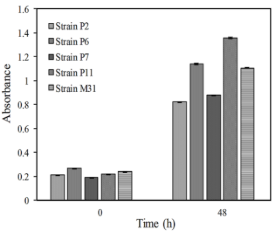
Figure 2. Growth of isolated yeast strains with sucro-se as carbon and energy source.
3.5 Ethanol tolerance of isolated yeasts
The response of the yeast strains to increasing ethanol concentrations in the culture medium was studied and the results are presented in Table 5.
Analysis of variance determined that ethanol concentration significantly influenced the growth of the yeast strains (p ≤ 0.05). Table 5 shows that yeast strains P2 and P7 exhibited tolerance up to an ethanol concentration of 4% (v/v), with 6% (v/v) of ethanol the growth of both strains was inhibited. P6 strain grew up to an ethanol concentration in the medium of 8% (v/v) and was completely inhibited with 12% (v/v). P11 strain proved to be more resistant to ethanol and was able to grow up to 10% of ethanol; with 12% (v/v) the growth decreased appreciably. M31 strain was the least tolerant and was inhibited when 4% (v/v) of ethanol was added to the medium. Ethanol production is limited by the inhibitory effect that ethanol itself exerts on the strain that produces it, affecting its growth and therefore the production of this metabolite.
Table 5
Growth of the isolated yeasts with different initial ethanol concentrations
Etanol % (v/v) | ΔAbs620 |
Strain P2 | Strain P6 | Strain P7 | Strain P11 | Strain M31 | Control |
0 | 1.23 ± 0.01 | 1.50 ± 0.020 | 1.09 ± 0.038 | 1.36 ± 0.015 | 1.36 ± 0.044 | 1.16 ± 0.008 |
4 | 0.88 ± 0.02 | 1.49 ± 0.036 | 0.85 ± 0.019 | 1.29 ± 0.019 | 0.19 ± 0.002 | 0.74 ± 0.010 |
6 | 0.21 ± 0.04 | 1.47 ± 0.051 | 0.22 ± 0.004 | 1.32 ± 0.021 | - | 0.64 ± 0.018 |
8 | - | 0.89 ± 0.007 | - | 1.30 ± 0.036 | - | - |
10 | - | 0.22 ± 0.013 | - | 1.12 ± 0.027 | - | - |
12 | - | - | - | 0.35 ± 0.080 | - | - |
Abs620 values of the culture, mean (n=3) ± SD. ΔAbs620= Absfinal – Absinicial. -: no growth.
Since alcohol is toxic to microorganisms, only small concentrations of it can accumulate during alcoholic fermentation, which depends on the tolerance of each yeast strain. By regulating the initial substrate concentration, an adequate fermentation yield can be maintained without causing excessive accumulation of ethanol in the medium, which would eventually create toxic conditions for the yeast [14, 24]. López-Álvarez (2007), concluded that ethanol concentrations of 10% or higher were toxic to four wild yeasts isolated from agave juice, considering that their growth was inhibited at those ethanol concen-trations. Ortiz-Samora et al. (2009) reported that four yeast strains isolated from natural sources (grape juice, sugarcane molasses and sugarcane juice) in Veracruz, Mexico, showed a strong inhibitory effect with ~ 6% (w/v) of ethanol.
3.6 Tolerance to sulfur dioxide of isolated yeasts
The tolerance to sulfur dioxide (SO2) of the yeasts is shown in Table 6. Table 6 shows that P2 and P7 strains could not grow in the presence of SO2. The rest of the yeast strains (P6, P11, M31 and the control) grew adequately in the presence of 65 mg/L of SO2, no significant difference was observed between these absorbance values and those of cultures in the absence of SO2. No yeast growth was observed in the culture medium containing 130 mg/L of SO2. During the winema-king process, SO2 is added as an antioxidant and antiseptic, so the strains P6, P11, and M31 can be used to carry out alcoholic fermentation considering they tolerate SO2 concentration of 60 mg/L, frequently used in winemaking (Voget et al., 2013; Miranda-Castilleja et al., 2015).
3.7 Alcoholic fermentation
The results of the alcoholic fermentations of the selected yeast strains and those of the commercial strain, in a semi-synthetic medium with 100 g/L of glucose, are shown in Figure 3. The ethanol produced by P6 and control strains was 4.5% (v/v) and the ethanol yield was 0.355 gethanol/gglucose (69.6% theoretical yield). Whereas strain P11 produced 5% (v/v) ethanol and a yield of 0.3945 gethanol/gglucose (77% theoretical yield). Ortiz-Zamora et al (2009), reported that yeast strains isolated from natural sources in Veracruz (México) showed a maximum yield of 0.46 gethanol/gglucose (90% theoretical yield).
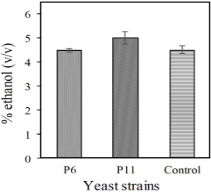
Figure 3. Ethanol produced by the yeast strains in a semi-synthetic medium.
4 Conclusions
In the present study 62 yeast strains were isolated from the spontaneous fermentation of native fruits musts of Misiones, Argentina (pitanga, black mulberry, cerella negra, jabuticaba, pindó and passion fruit). Of these isolated yeasts, 16 showed ethanol production capacity (26%). The isolates named as P2, P6, P7, P11 (isolated from pitanga) and M31 (isolated from black mulberry) were preselected for producing higher percentage of ethanol in a semi-synthetic medium containing 20 g/L glucose. Strains P6 and P11 were the most osmotolerant and grew with initial sugar concen-trations of 200 g/L. Regarding ethanol and SO2 tolerance, P6 and P11 strains grew with 8 and 10% (v/v) ethanol, respectively and in the presence of 65 mg/L SO2. The other yeast strains showed lower tolerance to these stress factors. Strains P6 and P11 achieved an ethanol yield that was 69.6 and 77% of the theoretical maximum yield, respectively.
According to the results of the present study, the strains P6 and P11 will be used in future expe-riments to produce regional alcoholic beverages from native fruits of Misiones province, with the goal of obtaining fruit wines with aromas and flavors distinct from those currently available on the market.
Table 6
Growth of isolated yeasts with different SO2 concentrations
SO2 (mg/L) | ΔAbs620 |
Strain P2 | Strain P6 | Strain P7 | Strain P11 | Strain M31 | Control |
0 | 1.23 ± 0.01 | 1.54 ± 0.025 | 1.09 ± 0.038 | 1.47 ± 0.015 | 1.36 ± 0.044 | 1.16 ± 0.008 |
65 | - | 1.47 ± 0.037 | - | 1.49 ± 0.068 | 1.33 ± 0.031 | 1.18 ± 0.013 |
130 | - | - | - | - | - | - |
Abs620 values of the culture, mean (n=3) ± SD. ΔAbs620= Absfinal – Absinicial. -: no growth.
References
Ajit, E. J., Dominic, D., Farook, F., Promod, A., Kumar, B., Blesy, V. J., Sabu, K. R., Rajesh, B. R. & Pratap C. R. (2018). Preparation of wine from fruits of Musa accuminata and Ananas comosus; its physico-chemical analyses and sensory evaluation. Integrative food, nutrition and metabolism, 5(6), 1-5. https://doi.org/10.15761/IFNM.1000232
Alezandro, M. R., Dubé, P., Desjardins, Y., Lajolo, F. M., & Genovese, M. I. (2013). Comparative study of chemical and phenolic compositions of two species of jaboticaba: Myrciaria jaboticaba and Myrciaria cauliflora. Food Research International, 54(1), 468-477. https://doi.org/10.1016/j.foodres.2013.07.018
Campos-Rodriguez, J., Acosta-Coral, K., Moreno-Rojo, C., & Paucar-Menacho, L. M. (2023). Maracuyá (Passiflora edulis): Compo-sición nutricional, compuestos bioactivos, aprovechamiento de subproductos, biocontrol y fertilización orgánica en el cultivo. Scientia Agropecuaria, 14(4), 479-497, 2023. https://doi.org/10.17268/sci.agropecu.2023.040
Canché-Collí, C., Barahona, F., Medina-Medina, L. A., & Canto, A. (2021). The effect of sugar concentration on growth of yeast associated to floral nectar and honey. Scientia fungorum, 52, e1288, 1-11. https://doi.org/10.33885/sf.2021.52.1288
Chacón-Ordóñez, T., & Esquivel-Rodríguez, P. (2013). Frutos tropicales como fuente de carotenoides: biosíntesis, composición, biodisponibilidad y efectos del procesamiento. Revista Venezolana de Ciencia y Tecnología de Alimentos, 4(1), 1-23.
Girardelo, J. R., Munari, E. L., Dallorsoleta, J. C., Cechinel, G., Goetten, A. L., Sales, L. R., Reginatto, H., Chaves, V. C., Smaniotto, F. A., Somacal, S., Emanuelli, T., Benech, J. C., Soldi, C., Winter, E., & Conterato, G. M. (2020). Bioactive compounds, antioxidant capacity and antitumoral activity of ethanolic extracts from fruits and seeds of Eugenia involucrata DC. Food Research International, 137, 109615. https://doi.org/10.1016/j.foodres.2020.109615
Guevara-Bravo, C., Acevedo, J., & Peláez Jaramillo, C. (2014). Aislamiento y caracterización de una levadura floculante para producir etanol del banano de rechazo. Biotecnología en el Sector Agropecuario y Agroindustrial, 12(2), 151-159.
Gundogdu M., Muradoglu, F., Gazioglu Sensoy, R. I., & Yilmaz, H. (2011). Determination of fruit chemical properties of Morus nigra L., Morus alba L. and Morus rubra L. by HPLC. Scientia Horticulturae, 132, 37-41. https://doi.org/10.1016/j.scienta.2011.09.035
Haro-Altamirano, J. P., Zambrano-Cárdenas, G. O., Cazorla-Vinueza, X. R., Soplín, H., & Garzón, R. (2021). Evaluation of the sustainability of family farming farms, of two eco-types of Yellow Pitahaya (Selenicereus megalanthus), and their by-product. Polo del Conocimiento, 6(12), 301-319.
Koyuncu, F., Çetinbaş, M., & Ibrahim, M. (2014). Nutritional constituents of wild-grown black mulberry (Morus nigra L.). Journal of Applied Botany and Food Quality, 87, 93-96. https://doi.org/10.5073/JABFQ.2014.087.014
López-Álvarez, J. A. (2007). Selección y manipulación genética de una levadura tolerante y sobreproductora de etanol, Tesis de Maestría, Universidad Michoacana de San Nicolás de Hidalgo, México.
Lorenzini, M., Simonato, B., Slaghenaufi, D., Ugliano, M., & Zapparoli, G. (2019). Assessment of yeasts for apple juice fermentation and production of cider volatile compounds. LWT - Food Science and Technology, 99, 224-230. https://doi.org/10.1016/j.lwt.2018.09.075
Loyo-Trujillo, N. A., Vivar-Vera, G., Sánchez-Bazán, I., Jiménez-Guzmán, J., & Del Ángel-Zumaya, J. A. (2020). Caracterización y elaboración de una bebida fermentada tipo vino de ciruela roja var. Black Amber. Coloquio de investigación multidisciplinaria, 8(1), 1842- 1848. http://dx.doi.org/10.23857/dc.v7i1.1691
Maidana, S. A., Butiuk, A. P., Zubreski, E. R., Hours, R. A., Brumovsky, L. A., & Martos, M. A. (2019). Production of an endopolygalacturonase from Wickerhanomyces anomalus with disintegration activity on plant tissues. Biocatalysis and Agricultural Biotechnology, 18, 101042. https://doi.org/10.1016/j.bcab.2019.101042
Malacrino, P., Tosi, E., Caramia, G., Prisco, R., & Zapparoli, G. (2005). The vinification of partially dried grapes: A comparative fermentation study of Saccharomyces cerevisiae strains under high sugar stress. Letters in Applied Microbiology, 40, 466-472. https://doi.org/10.1111/j.1472-765X.2005.01713.x
Martos, M. A., Zubreski, E. R., Combina, M., Garro, O. A., & Hours, R. A. (2013). Isolation of a yeast strain able to produce a polygalacturonase with maceration activity of cassava roots. Food Science and Technology, 33(2), 332–338. http://dx.doi.org/10.1590/S0101-20612013005000047
Mercado, L., Dalcero, A., Masuelli, R., & Combina, M. (2007). Diversity of Saccharomyces strains on grapes and winery surfaces: analysis of their contribution to fermentative flora of Malbec wine from Mendoza (Argentina) during two consecutive years. Food Microbiology, 24(4), 403-412. https://doi.org/10.1016/j.fm.2006.06.005
Miranda-Castilleja, D. E., Ortiz-Barrera, E., Arvizu-Medrano, S. M., Ramiro-Pacheco, J., Aldrete-Tápia, J. A., & Martínez-Peniche, R. A. (2015). Aislamiento, selección e identificación de levaduras Saccharomyces spp. nativas de viñedos en Querétaro, México. Agrociencia, 49(7), 759-773.
Ortiz-Zamora, O., Cortés-García, R., Ramírez-Lepe, M., Gómez-Rodríguez, J., & Aguilar-Uscanga, M. G. (2009). Isolation and selection of ethanol-resistant and osmotolerant yeasts from regional agricultural sources in México. Journal of Food Process Engineering, 32, 775-86. https://doi.org/10.1111/j.1745-4530.2008.00244.x
Peña, C., & Arango, R. (2009). Evaluación de la producción de etanol utilizando cepas recombinantes de Saccharomyces cerevisiae a partir de melaza de caña de azúcar. Dyna, 76(159), 153-161.
Rompato, K., Franco, R. R., Somoza, S. N., & Rompato, L. S. (2015). Composición nutricional de frutos de Syagrus romanzoffiana (pindó) nativos de Formosa- Argentina. Boletim do Centro de Pesquisa e Processamento de Alimentos, 33(2), 105-112. http://dx.doi.org/10.5380/cep.v33i2.47296
Sahana, G. R., Balasubramanian, B., Joseph K. S., Pappuswamy, M., Liu, W. C., Meyyazhagan, A., Kamyab, H., Chelliapan, S., & Joseph, B. V. (2024). A review on ethanol tolerance mechanisms in yeast: current knowledge in biotechnological applications and future directions. Process Biochemistry, 138, 1-13. https://doi.org/10.1016/j.procbio.2023.12.024
Santamaría, P., López, R., López, E., Garijo, P., & Gutiérrez, A. (2008). Permanence of yeast inoculates in the winery ecosystem and presence in spontaneous fermentations. European Food Research and Technology, 227(5), 1563-1567. https://doi.org/10.1007/s00217-008-0855-5
Suárez-Valles, B., Pando-Bedriñana, R., Fernández-Tascón, N., González-Garcia, A., & Rodríguez-Madrera, R. (2005). Analytical differentiation of cider inoculated with yeast (Saccharomyces cerevisiae) isolated from Asturian (Spain) apple juice. LWT - Food Science and Technology, 38(5), 455-461. https://doi.org/10.1016/j.lwt.2004.07.008
Voget, C., Broncompagno, N., Villa Monte, I., Romero, M., Velarde. I., Filleira, G. S., & Borrajo, A. (2013). Vinos de fruta en América. La Alimentación Latinoamericana, 306, 56-67.