Alternatives to pure chemical reagents: Use of soluble fertilizers in the
in vitro propagation of Musa AAB cv. Hartón
Luis Godoy-Montiel1 *; María Eugenia Romero-Román1; Nataly Herrera-Tamayo1
Ronald Oswaldo Villamar-Torres1; Seyed Mehdi Jazayeri2; Tefide Kızıldeniz3
- Facultad de Ciencias Pecuarias y Biológicas, Universidad Técnica Estatal de Quevedo, Quevedo 120509, Ecuador.
- ERIT PSII – Plant Science, Interactions and Innovation, Institut Agrosciences, Environnement, et Santé (AgES), Avignon Université, Avignon, France.
- Niğde Ömer Halisdemir University, Faculty of Agricultural Sciences and Technologies, Biosystem Engineering Department, 51240, Niğde, Turkiye.
ORCID de los autores:
L. Godoy-Montiel https://orcid.org/0000-0002-0551-9974 M. E. Romero-Román https://orcid.org/0000-0003-2665-5366
N. Herrera-Tamayo https://orcid.org/0000-0002-9949-2775 R. O. Villamar-Torres https://orcid.org/0000-0003-2511-1789
S. Mehdi Jazayeri https://orcid.org/0000-0003-2852-5063 T. Kızıldeniz https://orcid.org/0000-0002-5627-1307
ABSTRACT
In vitro propagation has emerged as a crucial technique in the propagation of plantain (Musa AAB cv. Hartón), a crop of high commercial demand in Ecuador, which allows the mass production of healthy and uniform plants, facilitating the rapid multiplication of cultivars with desirable characteristics, contributing significantly to crop health by eliminating pathogens and producing disease-free plants. To evaluate the use of soluble commercial fertilizers in the formulation of macronutrients in the in vitro propagation of plantain, the Student Test was applied with two treatments (chemical reagents and soluble fertilizers), each one including 10 observations. The corms were disinfected with 96% alcohol and a 1% sodium hypochlorite solution and cultured in MS medium. Ammonium nitrate, potassium nitrate, magnesium sulfate heptahydrate and potassium phosphate monobasic were used as soluble fertilizers, using molar equivalences to ensure the same amount of nutrients as the pure chemical reagents. The treatment with soluble fertilizers (Treatment 2) presented a higher percentage of phenolization (65%), fungal contamination (30%) and bacterial contamination (40%) compared to the treatment with chemical reagents. Bacterial contamination was controlled with the use of a bactericide. The use of highly soluble fertilizers promoted a faster phenological development, and after the breaking of apical dominance, the incidence of fungal contamination decreased, without presenting bacterial contamination. These findings suggest that soluble fertilizers may be a viable and effective alternative to pure chemical reagents in the in vitro propagation of Musa AAB cv. Hartón.
Keywords: In vitro culture; Musa paradisiaca var. Hartón; plant biotechnology; Soluble commercial fertilizers; plant health.
1. Introduction
Ecuador stands out as an agricultural country where the agribusiness sector serves as a fundamental pillar of the economy, with plantain (Musa AAB) being one of its most significant products. This crop, particularly the Hartón variety, is essential not only for food security but also for foreign exchange generation, as it constitutes one of the country's main sources of income (Rico et al., 2024). Moreover, the banana and plantain sectors have integrated technology and sustainability into production, aligning with circular economy models and promoting traceability throughout the value chain (Vergara-Romero et al., 2022). Plantain accounts for approximately 32% of the global trade of this fruit, and in Ecuador, around 350,000 hectares are cultivated, mainly in the coastal provinces of Manabí, Guayas, and El Oro (Cedeño-Aviles et al., 2021).
Despite its economic importance, plantain production faces several challenges, particularly related to diseases and pests that compromise plant health, reducing both yield and crop quality (Mwangi et al., 2023). Considering associated crops’ scenario, plantain is widely cultivated along with cocoa, both playing a key role in shaping agroecosystems, influencing the interaction dynamics between crops and their environment, thus, ensuring plantain crops remain disease-free is critical for sustainable production (Vera-Velez et al., 2024). In this context, in vitro propagation has emerged as a promising technique for the rapid and disease-free multiplication of plants, ensuring a more efficient and controlled production system capable of generating high-quality agronomic and phytosanitary plants (Hasnain et al., 2022).
Micropropagation is a key biotechnological tool in this process, that has been proven effective in the mass production of Musa AAB var. Hartón plantlets, utilizing techniques such as organo-genesis and somatic embryogenesis (Mongelos Franco et al., 2020). In vitro micropropagation is a reliable alternative to conventional banana and plantain propagation systems, enabling mass production of disease-free plantlets. Direct organogenesis is the main pathway for shoot proliferation, though its efficiency remains genotype dependent. Addressing key challenges, including tissue browning and microbial contamination, is crucial to optimizing in vitro multiplication protocols for Musa spp. (Agbadje et al., 2021). Furthermore, in vitro propagation requires an optimized culture medium that provides the essential nutrients for optimal plant development.
The in vitro culture medium is a critical component in micropropagation, consisting of mineral salts and phytohormones that promote plant tissue growth and regeneration. The selection of macronutrients, including nitrogen, phosphorus, potassium, calcium, and magnesium, is crucial to ensure adequate plant development (El-Aleem et al., 2016). Micropropagation is an adequate technique for producing high-quality plantain planting material on a commercial scale. Factors such as culture media composition, and acclimatization conditions significantly influence the success of in vitro multiplication (Waman & Bohra, 2019). The soluble commercial fertilizers have been evaluated as a viable alternative in fertilization plans (Ehmann et al., 2019) as well as to replace traditional mineral salts in culture media, demonstrating positive results in various plant species. These fertilizers offer advantages such as ease of application and cost-effecti-veness, making them an attractive option for in vitro agriculture.
In this regard, the use of soluble fertilizers for the formulation of in vitro culture media may represent an effective and economical solution for the propagation of Musa AAB var. Hartón. These fertilizers provide balanced and efficient nutrition, enhancing plant development without compro-mising quality or productivity (Malhotra, 2016). This study explores the impact of soluble commercial fertilizers on macronutrient formula-tion in the in vitro culture of Musa AAB var. Hartón, evaluating its effectiveness in improving plantlet propagation and production.
2. Methodology
Suckers of Musa AAB var. Hartón were used as plant material. These were obtained from productive plants displaying optimal physiological and phytosanitary conditions, identified in farms located in the Mocache area of Los Ríos province, Ecuador. The suckers were thoroughly washed with water and detergent to remove soil residues. The extracted corms were trimmed to a size of 2.5 cm in diameter and 4 – 5 cm in height, then washed with running water and disinfected with 96% ethanol for 10 minutes. Subsequently, they were immersed in a 1% sodium hypochlorite solution for another 10 minutes. The corms were then rinsed three consecutive times with distilled water to remove any residual sodium hypochlorite. For the preparation of culture media, distilled water was used, and the pH was adjusted to 5.6 using KOH and/or HCl. The medium was sterilized at 15 PSI and 121 °C. A total of 20 mL of culture medium was dispensed into each test tube, which was then sealed with Parafilm®. The explants were initially incubated in darkness for eight days, after which the incubation conditions were adjusted to 24 – 26 °C, 80% – 85% relative humidity, a light intensity of 30 µmol m⁻² s⁻¹, and a 16/8 h photoperiod for four weeks, until the shoots reached approximately 1 cm in length. At this stage, suitable shoots were obtained for in vitro multiplication of apical buds. The extraction and inoculation of the apical meristems were conducted under a laminar flow hood after disin-fection. The isolated meristems were cultivated in a modified Murashige and Skoog (MS) medium for in vitro establishment, supplemented with 2 mg/L BAP + 0.3 mg/L IBA. For the multiplication phase, the modified MS medium was supplemented with 4 mg/L BAP.
Characteristics and incorporation of soluble fertilizers
To replace macronutrients traditionally supplied as pure chemical reagents, highly soluble commercial fertilizers were used. A molar equivalence proce-dure was performed to ensure that the propagules received the same nutrient concentrations as those provided by chemical reagents (Table 1). The molecular weight of the reagent and the element, the percentage of the desired element in commercial fertilizers, and the total molecular weight were considered to determine the fertilizer amounts required to supply the recommended dose according to Murashige & Skoog (1962).
Growth and quality parameters in the replacement of chemical reagents with soluble fertilizers for in vitro propagation
During the initiation phase, after explant inoculation, data were collected on the evaluated treatments following the methodology described by El-Aleem et al. (2016). The general appearance of the explants was observed, with special attention to discoloration caused by phenolic compound accumulation due to oxidative stress. A 1 – 10 scale was used, classifying explants from least to most phenolized. Evaluations were performed at 15 and 30 days after inoculation. Likewise, periodic visual inspections were conducted under sterile conditions to detect any undesired growth. Contamination was recorded as 1 (presence) or 0 (absence), and the number of contaminated explants was expressed as a percentage of the total per treatment per pathogen. At the end of the cultivation period, the number of surviving explants was compared with the initial count to calculate the survival percentage.
Effect of apical dominance disruption on morphological and contamination variables
Apical dominance was disrupted at 45 days by making a vertical incision in the initial explant to halt radial growth and stimulate lateral bud activation. Each incised explant was transferred to a flask containing culture medium. Before and after this procedure, the following variables were assessed: The number of shoots per explant was recorded before the incision. Each explant was examined individually. Similarly, the diameter of each explant was measured using a caliper before the incision, ensuring accurate and detailed measurements. The length of each explant was recorded using a caliper before the incision, guaranteeing precision and the number of shoots per explant was recorded every week. Regarding to fungal and bacterial contamination in the medium after apical dominance disruption, visual inspections were conducted to detect fungal growth in the culture medium. Contamination was recorded as 1 (presence) or 0 (absence), and the number of contaminated explants was expressed as a percentage of the total per treatment. To achieve the established objectives, the Student test was applied, consisting of two treatments and 10 observations per treatment (Table 2), where each observation was considered an experimental unit.
Statistical analysis
To evaluate the results, the data were statistically processed using Student test analysis in the freely licensed statistical software Infostat.
Table 1
High-solubility fertilizers used to replace four chemical reagents comprising the macronutrients of the MS culture medium for the in vitro multiplication of Musa AAB var. Hartón
Common Name and Formula | (N-P-K-Ca-Mg-S) | Dose (mg/L) |
Ammonium Nitrate (NH₄NO₃) | 34.5-0-0-0-0-0 | 1.640 |
Potassium Nitrate (KNO₃) | 13.5-36.5-0-0-0-0 | 2.040 |
Magnesium Sulfate Heptahydrate (MgSO₄·7H₂O) | 0-0-0-0-9.8-13-0 | 372 |
Monobasic Potassium Phosphate (KH₂PO₄) | 0-52-34-0-0-0-0 | 170 |
Table 2
Description of the treatments to assess the effect of using soluble fertilizers as a replacement for pure chemical reagents in the formulation of macronutrients in the MS medium
Treatments | Description |
T1 (Chemical Reagents) | The macronutrients of the culture medium were added as pure chemical reagents, following the formulation recommendations of Murashige and Skoog. |
T2 (Soluble Fertilizers) | The macronutrients were added using soluble fertilizers as the source. |
- Results and discussion
Percentage of phenolization
Our findings demonstrate the dynamic nature of explant phenolization and the influence of the culture medium over time. At 15 days post-planting, the MS medium with pure chemical reagents showed a higher phenolization rate, suggesting that its components promoted phenolic compound synthesis or oxidation, while soluble fertilizers delayed phenolization, possibly due to their impact on nutrient availability and metabolic processes. However, by 30 days, the reversal in phenolization rates may be attributed to the soluble fertilizers improving the overall health of the explants, enhancing cellular processes and reducing phenolization. Despite this, no significant statistical differences between these two treatments (p ≥ 0.01) were observed, indicating that the medium composition had a minimal impact on phenolization under the experimental conditions.
Previous studies have reported varying results on explant phenolization. Justine et al. (2022) reported a significantly lower phenolization rate (6.25%) in banana explants cultured in different media for in vitro propagation. This stark difference may be attributed to the use of ascorbic acid and L-cysteine in their study, which were employed to prevent the phenolization of plant tissues. The addition of these compounds likely mitigated the oxidative processes that lead to phenolization. In contrast, our study did not utilize such compounds, which may explain the higher phenolization rates observed here.
Moreover, Permadi et al. (2023) informed the cultured plantain explants in darkness to minimize oxidation, resulted in the loss of media compounds after the use of desinfectants, which is consistent with our findings that explants can still undergo phenolization despite attempts to limit oxidation. These studies collectively underscore the comple-xity of phenolization in plant tissue culture and suggest that multiple factors, including medium composition, the presence of antioxidants, and light exposure, play crucial roles in regulating phenolic compound accumulation during in vitro propagation.
Percentage of contamination caused by fungi and bacteria
The percentage of phenolization in the explants was evaluated at two different time points: 15 and 30 days after planting The evaluation of fungal contamination in the MS medium for the propagation of Musa AAB var. Hartón revealed that the treatment with pure chemical reagents (T1) presented a fungal contamination rate of 10%, while the treatment with highly soluble fertilizers (T2) recorded a significantly higher contamination rate of 30%. The presence of microorganisms (Figure 1 A-B) was confirmed by microscopical observation. However, statistical analysis indicated that there were no significant differences (p ≥ 0.01) between the two treatments.
Regarding bacterial contamination, the MS medium with pure chemical reagents (T1) exhibited a bacterial contamination rate of 30%, while the treatment with highly soluble fertilizers (T2) showed a higher incidence of bacterial contamination, exceeding 40%. Again, statistical analysis revealed no significant differences between the treatments assessed. It is notable that during the study, Gentamax 280 was applied to both treatments to control microbial contamination. Despite its use, the contamination levels observed remained relatively consistent across treatments.
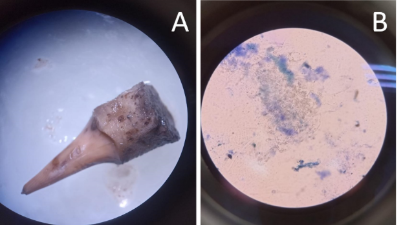
Figure 1. Presence of bacteria in the explant of Musa AAB var. Hartón. A: Bacteria present in explant grown in a culture medium with soluble fertilizers; B: Bacteria of the genus Coccus viewed under the microscope after being stained with Gram staining.
The presence of bacteria in an explant of Musa AAB var. Hartón (Figure 1), which was cultured in a medium containing highly soluble fertilizers as a substitute for pure chemical reagents (T2). After applying Gram staining, the bacteria were identified under the microscope as Gram-positive and spherical (cocci), arranged in irregular clusters. Based on these results, highly soluble fertilizers may be promoting the growth of Gram-positive bacteria in explants of Musa AAB var. Hartón.
Contamination rates are influenced by several factors, including the origin of plant material, the disinfection protocol employed, the medium composition, and the environmental conditions during culture establishment. Our result was similar with the findings of Mongelos Franco et al. (2020) who evaluated different concentrations of sodium hypochlorite for the disinfection of banana meristems for in vitro propagation. Their observation suggests that, despite efforts to control contamination using chemical agents, complete sterilization may not always be achieved, particularly in cases involving fungal and bacterial pathogens. Santos et al. (2020) reported a higher average contamination rate of between 16.5% and 49.7%, as the explants in their study were exposed to disinfecting agents for a shorter period. The differing results may be attributed to variations in disinfection protocols and exposure times, which can significantly influence the success of sterilization procedures.
In another study by Verde et al. (2022), the culture medium employed, which contained activated charcoal, yielded a lower contamination rate of 15%. This study suggested that the lower contamination rate could be related to the origin of the plant material, as it was derived from field-grown material. In contrast, the material used in this study came from more controlled environments, which may have led to higher microbial loads on the explants, thus increasing contamination rates. Moreover, Eliwa et al. (2024) observed a high incidence of contamination in their study, where most explants became contaminated despite testing different disinfection protocols. The high contamination rate was likely due to the inadequacy of the cleaning process to remove organic material that could harbor microbial loads, which may not have been effectively eliminated during the disinfection process. Similarly, Ancasi-Espejo et al. (2016) pursued to determine the optimal culture medium for the establishment phase of in vitro banana propagation. Their study found that fungal contamination occurred at a rate of 11.1% (4/36 explants) at 30 days, while bacterial contamination was found in 16.7% (6/36 explants) of the explants. This data highlights that microbial contamination, particularly fungal and bacterial, is a common challenge during the establishment phase of in vitro propagation. In contrast, Cabral-Miramontes et al. (2022) reported a lower contamination rate when propagating apple trees in vitro, with an average contamination rate of 31.7% due to unidentified fungi and bacteria. This was attributed to the use of wild donor plants that lacked strict sanitary conditions, demonstrating that the source of plant material plays a significant role in the success of in vitro propagation.
Survival percentage
Regarding the survival percentage of explants, the Musa AAB var. Hartón treated with pure chemical reagents (T1) exhibited the highest survival rate, which reached 90%. In contrast, explants cultured with a medium that used highly soluble fertilizers as substitutes for the pure chemical reagents (T2) showed a significantly lower survival rate, recorded at 70% (Figure 2). This result suggests that the use of pure chemical reagents has a more favorable impact on the survival of explants in comparison to the alternative medium with highly soluble fertilizers.
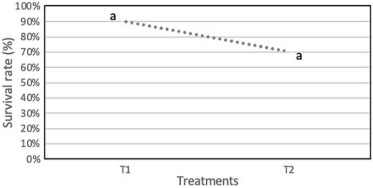
Figure 2. Survival rate of plantain explants using fertilizers as substitutes for pure chemical reagents.
Our findings in the shoots emission is similar to the report done by García et al. (2021), the micropropagation of the plantain cultivar Enano Guantanamero at 21 days in the multiplication phase yielded an average of 1.95 shoots per explant. This was achieved using 4 mg/L of BAP combined with 10 mg/L of a commercial product that enhances growth. This hormonal synergy appears to stimulate the induction of lateral shoots while maintaining the stability and growth of the explants, thereby optimizing the overall micropropagation process.
Additionally, our findings indicate that soluble fertilizers could play a key role in enhancing the in vitro growth conditions of plantain explants. The substitution of pure chemical reagents with soluble fertilizers may create a more balanced nutrient environment, contributing to better plantlet development and shoot formation. This approach could offer significant improvements in plantain micropropagation protocols, particularly in terms of cost-effectiveness and the reduction of chemical input.
On the other hand, the findings of shoot survival percentages is aligned with the work of Sosa-Amay et al. (2017) who reported a 100% survival rate when using conventional fertilizers as substitutes for mineral salts. However, the results presented in this study indicate a lower survival rate, suggesting that the substitution of pure chemical reagents with highly soluble fertilizers may not provide the same level of efficiency in promoting explant survival. This discrepancy might be attributed to differences in experimental conditions, such as the concentration and type of fertilizers used, or other environmental factors that may have influenced the performance of the explants in both studies.
Phenological growth
The time required (in days) for the explants to complete each stage of in vitro phenological development was analyzed (Figure 3). In T1, growth and color change in non-functional leaves occurred 31 days after explant planting, while in T2, this process was observed at 23 days. The appearance of shoots was first recorded in T2, 35 days after explant planting, in contrast to T1, where shoots appeared after 59 days. T2 exhibited earlier phenological development compared to T1, as evidenced by the shorter time for growth and color change in non-functional leaves and shoot emergence (Figure 4). In this regard, Hassen et al. (2022) observed a color change and an increase in meristem size 21 days after in vitro establishment. In that study, coconut water was used in the culture medium, which is known for its plant growth-promoting properties and its content of organic compounds such as cytokinins. Compared to the results of the present study, this difference could be attributed to the use of this phytohormone, which possesses growth-promoting characteristics.
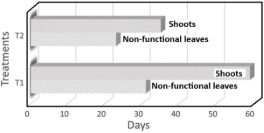
Figure 3. Duration of each in vitro growth stage of the Musa AAB explants.
Number of shoots per explant prior to the apex dominance disruption
The presence of lateral shoots was observed in the treatment that incorporated soluble fertilizers into the MS medium, replacing pure chemical reagents. In this treatment, the average number of shoots per explant was 1.14, whereas no shoots were observed in the unmodified MS medium (Table 3). These findings highlight the impact of soluble fertilizers in promoting shoot development, suggesting that the modifications to the MS medium may support better in vitro growth conditions for plantain micropropagation.
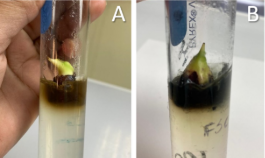
Figure 4. Development of Non-Functional Leaves in plantain explants. A: Green coloration of non-functional leaves in culture medium with chemical reagents; B: Green coloration of non-functional leaves in culture medium with soluble fertilizers.
Number of shoots per explant prior to the apex dominance disruption
The presence of lateral shoots was observed in the treatment that incorporated soluble fertilizers into the MS medium, replacing pure chemical reagents. In this treatment, the average number of shoots per explant was 1.14, whereas no shoots were observed in the unmodified MS medium (Table 3). These findings highlight the impact of soluble fertilizers in promoting shoot development, suggesting that the modifications to the MS medium may support better in vitro growth conditions for plantain micropropagation.
Length and diameter of explant PRD (cm)
The length of the explant PRD is not affected by the nutrient source, whether pure chemicals or soluble fertilizers, used in the MS medium for propagation of Hartón plantain. Table 3 shows that when chemical reagents (T1) were used, an average length of 3.01 cm was recorded, which is significantly higher than the average observed when soluble fertilizers were used (2.16 cm). In a similar study, Arbeláez et al. (2016) employed a disinfection method for in vitro propagation consisting of a solution of 1.5% Extran soap, 2% sodium hypochlorite, 70% alcohol, and citric acid at a concentration of 250 mg/200 ml, and they reported a length of 1 to 2 cm in Hartón plantain explants. On the other hand, Agbadje et al. (2021) reported a shoot length of 1.8 cm, a growth that may be attributed to an insufficient concentration of mineral salts, as they used Sulpomag as a partial substitute.
When analyzing the diameter of the explant PRD, the applied treatment does not significantly affect its growth; rather, both nutrient sources promote similar development of explant diameter during this stage (Table 3). Bošnjak et al. (2021) in their research on in vitro cultivation of functional fruits, the reproduction quality, implying a better plant vigor with a reduced vitrification, was obtained on the MS medium. A concentration optimization of individual mesocomponents (i.e., of the macro-elements) and their chemical forms (FeEDDHA) contributed to their improved adoption and consequently to the stress suppression due to an explant transplantation. Additionally, the use of Murashige and Skoog (MS) and Woody Plant Medium (WPM) supplemented with phytohor-mones allowed Cabral-Miramontes et al. (2022) to obtain explants with a diameter of 1.77 cm. However, Zribi et al. (2015) reported explant diameters ranging from 0.20 to 0.26 cm using the same medium supplemented with 6-benzyl-aminopurine (BAP) and gibberellic acid, values lower than those obtained in the present study.
Number of shoots, percentage of fungal and bacterial contamination after apical dominan-ce disruption (PRD)
Regarding the number of shoots per explant obtained after the apical dominance disruption cut in Hartón plantain explants and the percentage of contamination caused by fungi and bacteria, no significant differences (p ≥ 0.01) were observed in the number of shoots per explant after the apical dominance disruption, nor in the percentage of contamination caused by fungi and bacteria among the evaluated treatments. However, both fungal and bacterial contamination were detected in all treatments, including those in which the original MS medium was used and those where soluble fertilizers replaced the medium after the apical dominance disruption process.
Additionally, as noted by Permadi et al. (2024) The accumulation of phenolic compounds released by explants led to tissue necrosis and reduced viability, especially in media with alternative fertilizers, as a defense response to stress that ultimately hindered shoot survival. Additionally, fungal and bacterial contamination in both standard and modified MS media posed a persistent challenge, significantly affecting the development of banana explants. Bacterial contamination, particularly by Gram-positive Bacillus species, was characterized by a creamy, milky, and slightly mucous appearance around the explants. This contamination pattern is similar to that described by Hassen et al. (2022), who reported bacterial contamination rates of 70%, especially in explants from plants located in flooded areas. In their study, partial control of contamination (50% – 70%) was achieved by using chloramphenicol and rifampicin as antibiotic treatments.
Table 3
Average of three morphological parameters of Hartón plantain explants after the disruption of apical dominance propagated under two conditions of the MS medium
Treatment | Number of PRD Shoots | Explant Length (cm) | Explant Diameter (cm) |
Treatment 1 | 0.00 | 3.01 | 1.55 |
Treatment 2 | 1.14 | 2.16 | 1.26 |
Significancy level | *** | *** | ns |
Data are expressed as mean. ***= Highly significant differences at p ≤ 0.01 level. ns= No significant differences at p > 0.01 level.
In contrast, the study by Arbeláez et al. (2016) reported lower contamination rates, with a 2% contamination in the culture medium and 30% in the explants, when using a combination of disinfection methods, including 5% sodium hypochlorite, BAP, ascorbic acid, streptomycin, and Tween. The discrepancy in contamination levels between studies may be attributed to differences in environmental conditions, disinfection protocols, and the genetic characteristics of the banana varieties used.
The widespread contamination observed in our study emphasizes the need for more effective sterilization and disinfection methods in plantain micropropagation. The application of more stringent surface sterilization techniques or the use of specific antimicrobial agents might be necessary to reduce contamination and improve the in vitro performance of plantain explants. The microbial contamination was observed to cover the entire surface of the culture medium, which hindered the proper development of the explants. This resulted in the eventual death of the explants (Figure 5). The contamination caused by fungi and bacteria negatively affected the in vitro growth, leading to poor tissue differentiation and a reduction in the overall success rate of the propagation process.
4. Conclusions
This study, on the substitution of pure chemical reagents with commercial soluble fertilizers in the formulation of macronutrients in the Murashige and Skoog medium for in vitro culture of Musa AAB var. Hartón, demonstrated its feasibility for the micropropagation of this species. The absence of significant differences between the groups evaluated highlights its potential to optimize costs and improve efficiency in micropropagation, representing an alternative in plantain research and production.
The results related to growth and quality parameters show that there were no statistical differences in phenolization between the use of the unmodified MS medium (T1) and the one in which the reagents were replaced by soluble fertilizers (T2), although the former suggested better explant survival. However, high levels of fungal and bacterial contamination were observed in T2, with the presence of Gram-positive bacteria adhering to the explants. The use of highly soluble fertilizers allowed for faster phenological development, with the growth and color change of non-functional leaves and the appearance of shoots occurring in a shorter time frame.
Before the break in apical dominance, the morphological variables that showed statistical differences were the number of shoots and explant length. After the break in dominance, no statistical differences were found, although T2 showed a higher number of shoots. In this treatment, where highly soluble fertilizers were used, the incidence of fungal contamination was lower, and no bacterial contamination was observed.
The hypothesis is accepted, as the substitution of chemical reagents with commercial soluble fertilizers in the Murashige and Skoog medium for the micropropagation of Musa AAB var. Hartón is viable. Although no significant differences were found in phenolization between treatments T1 and T2, the higher fungal and bacterial contamination in T2 suggests that there may be differences in the quality and effectiveness of both treatments.
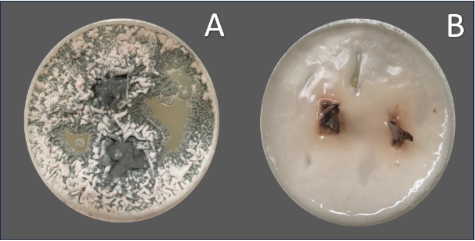
Figure 5. Culture medium of Musa AAB var. Hartón showing contamination detrimental to the development of the plantain buds. A: Fungal contamination with intense growth over the culture medium; B: Severe bacterial contamination causing explant death.
Acknowledgement
To the Universidad Técnica Estatal de Quevedo for partially supporting the fundings of this research through the Competitive Fund for Scientific and Technological Research (FOCICYT) Seventh Calling for Projects.
References
Abd El-Aleem, W. H., Ramadan, M. E., & Shalaby, O. A. (2016). Effect of magnesium fertilization on growth, yield, chemical composition and essential oils of some new cultivars of parsley under sinai conditions. Egyptian Journal of Desert Research, 66(2), 267–286. https://doi.org/10.21608/ejdr.2016.6501
Agbadje, E. T. A. E., Agbidinoukoun, A., Zandjanakou-Tachin, M., Cacaï, G. T. H., & Ahanhanzo, C. (2021). Mass Production of Bananas and Plantains (Musa spp.) Plantlets through in vitro Tissue Culture Partway: A Review. European Journal of Biology and Biotechnology, 2(4), Article 4. https://doi.org/10.24018/ejbio.2021.2.4.229
Ancasi-Espejo, R. G., Montero-Tonconi, J. R., Ferreira-Castedo, N. J., & Muñoz-Guzmán, I. (2016). Determinación un mejor medio de cultivo en la fase de establecimiento para la propagación in vitro de plátano (Musa paradisiaca L). Journal of the Selva Andina Research Society, 7(2), 104–111.
Arbeláez, L. M. A., Montoya, J. L., & Saavedra, S. A. R. (2016). Assessment protocols for the establishment and disinfection in vitro meristem of banana Musa spp. Vitae, 23, S391.
Arun Waman, A., & Bohra, P. (2019). Factors Governing Success in Shoot Tip Culture of Bananas with Special Reference to Mixed Genomic Groups: An Overview. Erwerbs-Obstbau, 61(1), 9–21. https://doi.org/10.1007/s10341-018-0391-9
Bošnjak, D., Marković, M., Agić, D., Vinković, T., Tkalec Kojić, M., Ravnjak, B., & Stanisavljević, A. (2021). The Influence of Nutrient Media Modification on the Morphological Parameters in Raspberry (Rubus idaeus L.) Micropropagation in the Liquid and Semi-solid Media. Poljoprivreda, 27(1), 22–29. https://doi.org/10.18047/poljo.27.1.3
Cabral-Miramontes, J. P., Chávez-Simental, J. A., Pulido-Díaz, C., González-Portillo, M., Goche-Télles, J. R., Barragán-Hernández, V. M., Cabral-Miramontes, J. P., Chávez-Simental, J. A., Pulido-Díaz, C., González-Portillo, M., Goche-Télles, J. R., & Barragán-Hernández, V. M. (2022). Propagación in vitro de manzano a partir de embriones cigóticos maduros. Revista mexicana de ciencias agrícolas, 13(4), 603–616. https://doi.org/10.29312/remexca.v13i4.2164
Cedeño-Aviles, J., Avilés, D. F. V., Guerrero, F. C., & Vergara, J. L. T. (2021). Resiliencia de dos sistemas de producción de musáceas en dos zonas del trópico ecuatoriano. Revista Ciencia y Tecnología, 14(2), 17–26.
Ehmann, A., Bach, I.-M., Bilbao, J., Lewandowski, I., & Müller, T. (2019). Phosphates recycled from semi-liquid manure and digestate are suitable alternative fertilizers for ornamentals. Scientia Horticulturae, 243, 440–450. https://doi.org/10.1016/j.scienta.2018.08.052
Eliwa, G. I., El-Dengawy, E.-R. F., Gawish, M. S., & Yamany, M. M. (2024). Comprehensive study on in vitro propagation of some imported peach rootstocks: In vitro explant surface sterilization and bud proliferation. Scientific Reports, 14, 5586. https://doi.org/10.1038/s41598-024-55685-3
García, M. B., Avalos, D. M. R., Mojena, A. F., Cuba, B. G. G., Borges García, M., Reyes Avalos, D., & Frías Mojena, A. (2021). Micropropagación del plátano cultivar enano guanta-namero (Musa spp., aab) con el empleo del pectimorf® micropropagation of enano guantanamero platain cultivar (musa spp., aab) with the use of the pectimorf®.
Hasnain, A., Naqvi, S. A. H., Ayesha, S. I., Khalid, F., Ellahi, M., Iqbal, S., Hassan, M. Z., Abbas, A., Adamski, R., Markowska, D., Baazeem, A., Mustafa, G., Moustafa, M., Hasan, M. E., & Abdelhamid, M. M. A. (2022). Plants in vitro propagation with its applications in food, pharmaceuticals and cosmetic industries; current scenario and future approaches. Frontiers in Plant Science, 13, 1009395. https://doi.org/10.3389/fpls.2022.1009395
Hassen, N. I., Badaluddin, N. A., Mustapha, Z., & Zawawi, D. D. (2022). Identification and prevention of microbial contaminants in musa paradisiaca tissue culture. Malaysian Applied Biology, 51(5), 129–143.
Justine, A. K., Kaur, N., Savita, & Pati, P. K. (2022). Biotechnological interventions in banana: Current knowledge and future prospects. Heliyon, 8(11), e11636. https://doi.org/10.1016/j.heliyon.2022.e11636
Malhotra, S. K. (2016). Water soluble fertilizers in horticultural crops—An appraisal. The Indian Journal of Agricultural Sciences, 86(10), 1245–1256.
Mongelos Franco, Y., Mussi, C. E., Duarte Ovejero, N. N., & Díaz Lezcano, M. I. (2020). Protocolo de desinfección para establecimiento in vitro de meristema apical de banano Musa spp. CEDAMAZ, 10(2), 47–50.
Mwangi, R. W., Mustafa, M., Charles, K., Wagara, I. W., & Kappel, N. (2023). Selected emerging and reemerging plant pathogens affecting the food basket: A threat to food security. Journal of Agriculture and Food Research, 14, 100827. https://doi.org/10.1016/j.jafr.2023.100827
Permadi, N., Akbari, S. I., Prismantoro, D., Indriyani, N. N., Nurzaman, M., Alhasnawi, A. N., Doni, F., & Julaeha, E. (2024). Traditional and next-generation methods for browning control in plant tissue culture: Current insights and future directions. Current Plant Biology, 38, 100339. https://doi.org/10.1016/j.cpb.2024.100339
Permadi, N., Nurzaman, M., Alhasnawi, A. N., Doni, F., & Julaeha, E. (2023). Managing lethal browning and microbial contamination in Musa spp. tissue culture: Synthesis and perspectives. Horticulturae, 9(4), Art. 4. https://doi.org/10.3390/horticulturae9040453
Rico, L. J. M., Arias, L. M. A., & Aguirre, J. C. L. (2024). Effects of Fertilization and Planting Distance on Growth Parameters of Musa AAB Simmonds. Journal of Hunan University Natural Sciences, 51(9).
Santos, M. M. dos, Cezario, L. F. C., Simões, I. M., Baptista, J. O., Araujo, C. P. de, Mello, T. de, Mayard, H., Gonçalves, E. de O., Fontes, M. M. P., Schmildt, E. R., Lopes, J. C., Caldeira, M. V. W., & Alexandre, R. S. (2020). Disinfection protocol and in vitro germination of seeds of Dalbergia nigra. CERNE, 26, 238–246. https://doi.org/10.1590/01047760202026022714
Sosa-Amay, R., Rojas-Idrogo, C., & Delgado-Paredes, G. E. (2017). Propagación in vitro de camote [Ipomoea batatas (L.) Lam.] en medio de cultivo suplementado con fertilizantes convencionales. Avances En Investigacion Agropecuaria, 21(2).
Vera-Velez, R., Ramos-Veintimilla, R., & Grijalva-Olmedo, J. (2024). Optimizing Pathogen Control through Mixed Cocoa–Plantain Agroecosystems in the Ecuadorian Coastal Region. Agronomy, 14(6), Article 6. https://doi.org/10.3390/agronomy14061107
Verde, D. dos S. V., Mendes, M. I. de S., Souza, A. da S., Rabêlo, J. S., Pinto, C. R., Nobre, L. V. C., & Ledo, C. A. da S. (2022). Activated charcoal in the control of oxidation and in vitro growth of Dioscorea spp. Nodal segments. Acta Scientiarum. Biological Sciences, 44, e61950. https://doi.org/10.4025/actascibiolsci.v44i1.61950
Vergara-Romero, A., Garnica-Jarrin, L., Armas-Ortega, Y., & Pozo-Estupiñan, C. (2022). Relationship between Corporate Social Responsibility, Assets and Income of Companies in Ecuador. 125–137.
Zribi, I., Bayoudh, C., & Haouala, R. (2015). In vitro regeneration of the medicinal plant Cassia absus L. The Journal of Horticultural Science and Biotechnology, 90(1), 14–19.